2024-11-3017:53 Status:Complete Tags: Basics of quantum mechanics
Conjugation Definition
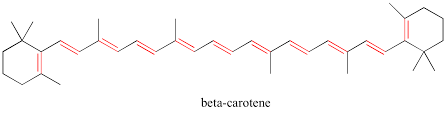
Pi conjugated systems such as benzene, and beta-carotene alternate between single bond, multi-bond, single bond, … () An example of this is in beta-carotene:
1,3-Butadiene, vs. 1,4-Pentadiene
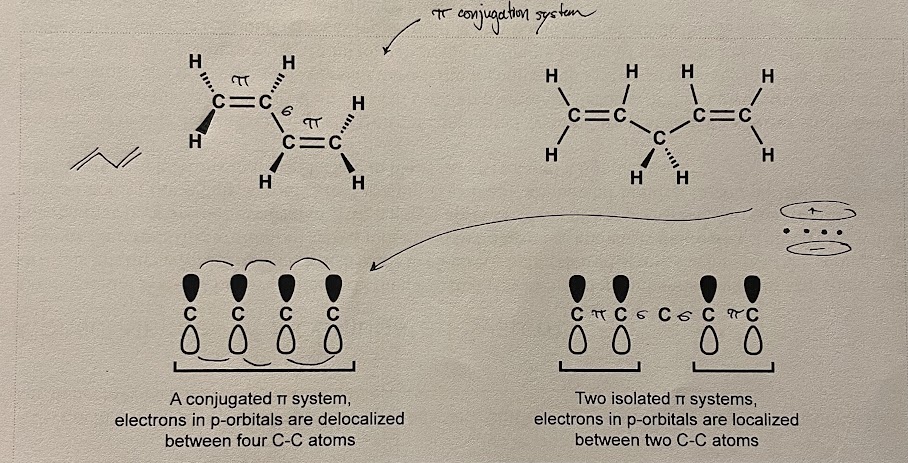
In 1,3-Butadiene, there is an alternating bond where each carbon atom has hybridization. This suggests that there is an unhybridized orbital for all carbon atoms on the same plane - oriented with the internuclear axis. This phenomenon is conjugation (linking). When conjugation extends past two atoms, it is extended conjugation. If there is a separation in orbitals and only two conjugated atoms, (take 1,4-pentadiene for example) then the conjugated atoms are isolated. 1,4-pentadiene happens to have two of them.
Consequences of Conjugation
When adjacent orbitals interact with each other, the electron initially in one orbital becomes delocalized, and “shared” across all atoms in the conjugation.
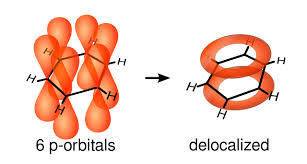
Understanding the Bonding in 1,3-Butadine
VBT describes the framework and MOT describes the framework of a molecule As electrons in orbitals do not interact with UV/visible light, they don’t contribute to the presence or absence of color. Thus, VBT is used to describe color of that comes from the delocalized electrons. Determining the number, type, and relative energies of the molecular orbitals that make up the framework involves 5 steps:
- Identify the number of atomic orbitals and their orientation (e.g. all the carbon atoms in 1,3-butadiene are coplanar, and hybridized)
- Identify the number of molecular orbitals that will be formed. (e.g. in 1,3-butadiene, each carbon atom has one orbital each: four molecular orbitals will form) Additionally, for every bonding orbital, there is an antibonding orbital. (… there will be 2 bonding and 2 antibonding orbitals in 1,3-butadiene).
- Draw the molecular orbitals. (this is similar to n=4, 3, 2, 1 particle in a 1-D box wavefunctions). For 1,3-butadiene:
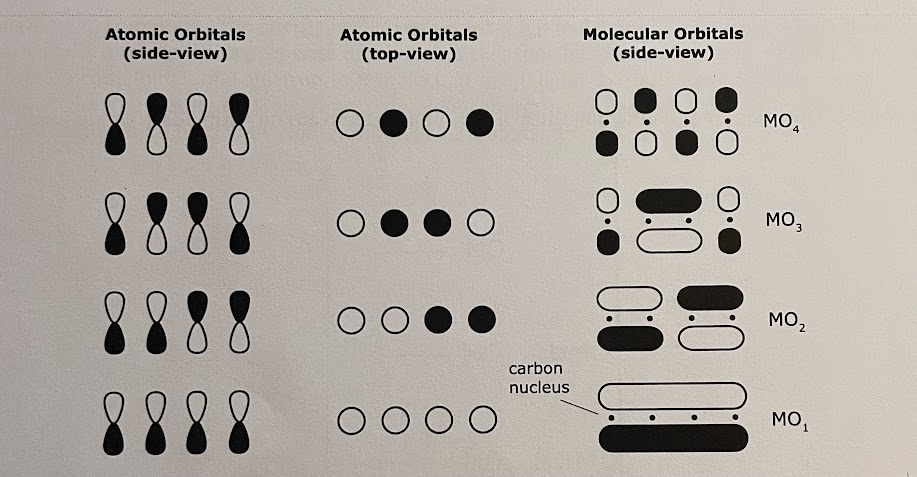
- Determine the relative energies of the molecular orbitals and whether they are bonding or antibonding orbitals. (Recall the increase # of nodes increases the energy. Because orbital energy is degenerate, they must all possess the same amount of energy) Notice that the higher the “n” of the orbital, the higher energy it has; and that antibonding orbitals have more energy than bonding .
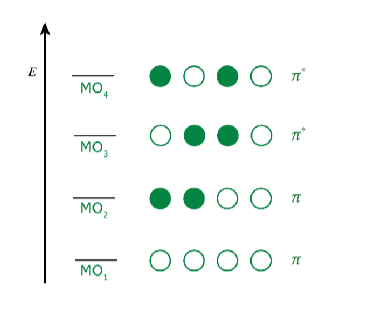
- Determine the number of electrons in the conjugated system and place them into the molecular orbitals.
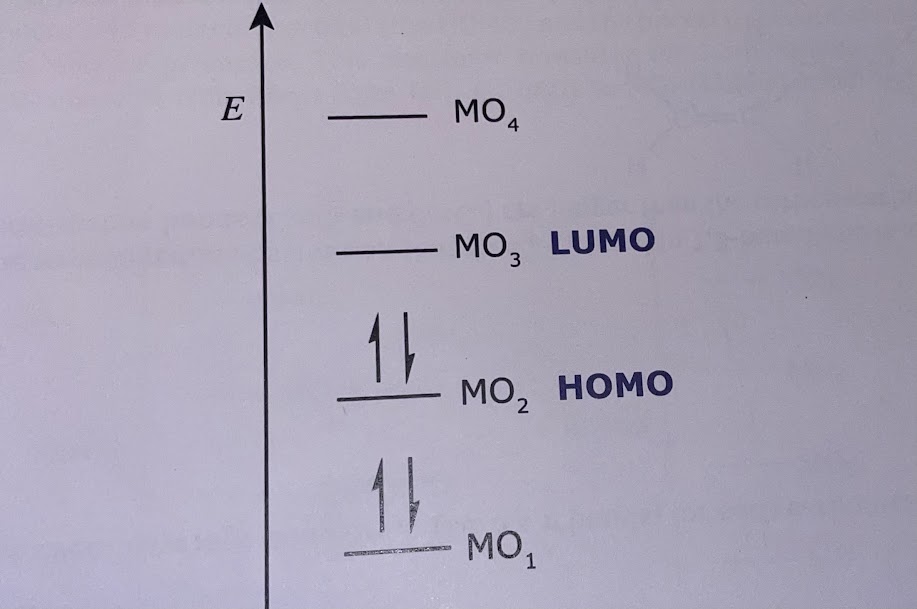
Using Particle-In-a-Box Model to Understand Absorption Process in Conjugated Systems
Similar to the excitation of an electron in a typical model, if the energy of an incident photon is equivalent to the energy required to excite an electron in the HOMO to the LUMO, then a specific wavelength is absorbed.
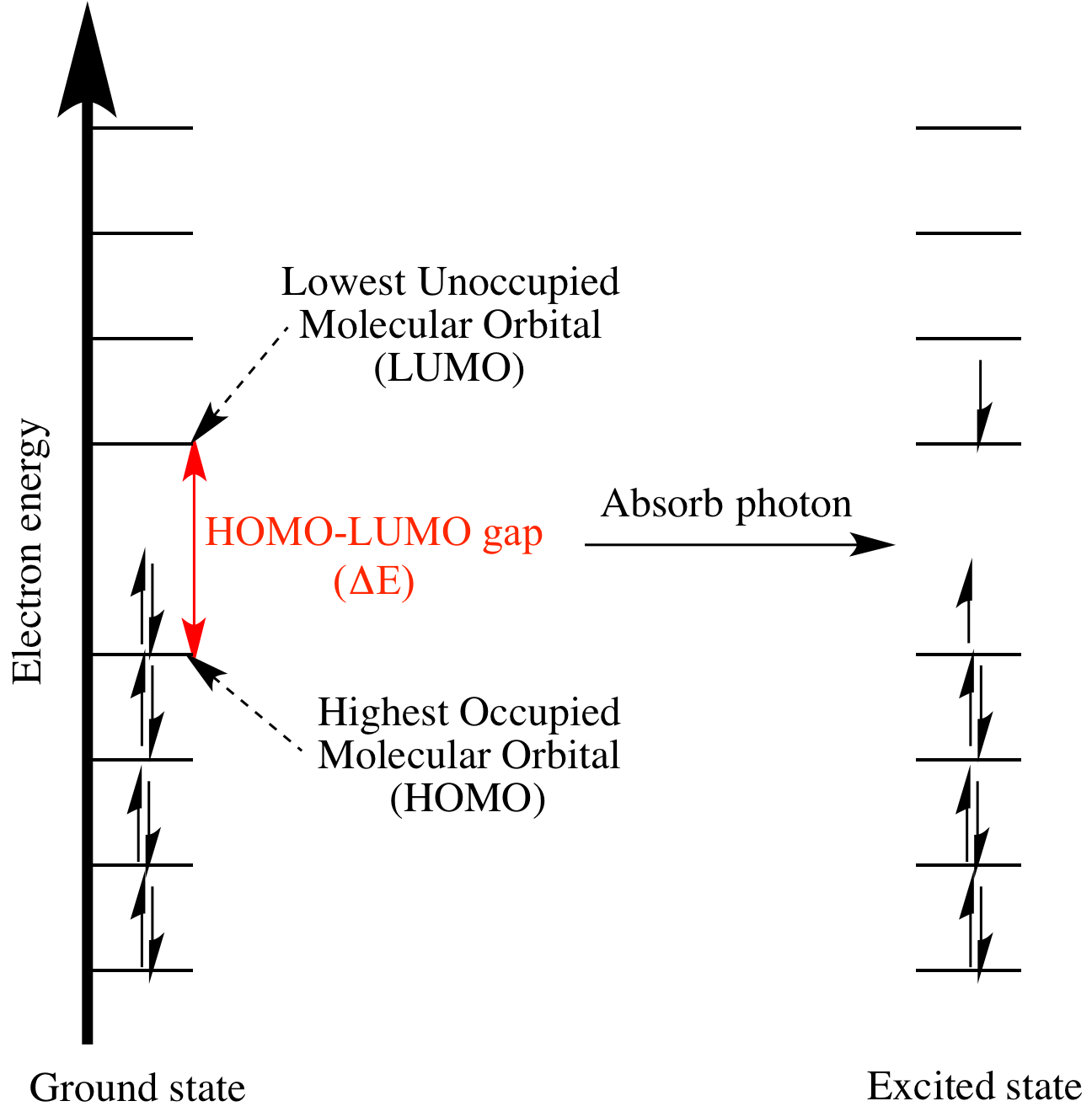
To implement the models for 1,3-butadiene, = length of carbon chain. For 1,3-butadiene, HOMO: , LUMO: . Use: to find the energy, length of a molecule, HOMO and LUMO of a molecule.
Colors of Molecules
If the wavelength of light is absorbed and in the visible region of the electromagnetic spectrum, the chemical will appear colored to our eyes. The most common process, reflection goes as follows:
- A light source generates light and is incident on some object.
- Light that is not absorbed by an object is reflected or passes through the object.
- Structures in the eye’s retina absorb the reflected light.
- Electrical signals are transmitted and interpreted by the brain.
The chemical structures in our eyes that allow us to see color are opsins (proteins in the retina) in two types of photoreceptors: rods and cones. Rods allow for low light vision, and Cones allow for colored vision. Opsins contain a chromophore which are conjugated components of a chemical structure that can absorb or emit light. Variation in opsins producing rhodopsin that absorb different wavelengths allow humans to see red, green and blue = trichromatic vision. - 3 opsin types.
- Protanopia = lack of red opsin production; difficulty distinguishing between red to green
- Deuteranopia = lack of green opsin production; difficulty distinguishing between red to green
- Tritanopia = lack of blue opsin production; difficulty distinguishing between yellow and green from blue.
- Tetrachromatic vision is rare but allows people to see more colors.
Subtractive Color Theory
To predict what color an object is we can figure out what wavelengths are absorbed and use the color wheel to determine what remaining light there is (assuming white light is incident). Materials that absorb all (visible) wavelengths appear black, materials that reflect all light appear their incident color (if white is shown, white is reflected and object is observed to be white).
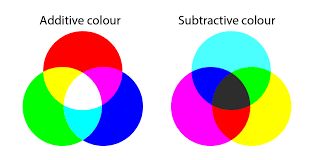 (If cyan is absorbed, then the object will appear red, and vice versa).
(If cyan is absorbed, then the object will appear red, and vice versa).
The broader the energy difference in a conjugated system, the wider the range of absorption. Take the maximum analytical wavelength of beta-carotene:

There are a total of 22 electrons in beta-carotene where the LUMO is , and the HOMO is .
Dyes
By changing the length and bond order of conjugated systems, the color of a synthetically produced dye can be changed.