2024-07-0914:08 Status:IBnotes
Background
The Nucleus of the Atom
Protons and neutrons are called nucleons because they are both components of nuclei. Three numbers describe the composition of the nucleus.
Atomic Number: Z = The number of protons in the nucleus Neutron Number: N = The number of neutrons in the nucleus Atomic Mass Number, (Mass number): A = the number of nucleons in the nucleus
A specific element is indicated using the symbol as shown on the periodic table along with both atomic number and the mass number. For example, carbon - 12 would be as shown: . For the element X with the number A and atomic number Z the symbol would be
Isotopes
Many elements have two or more isotopes, which are forms of elements that have the same amount of protons, Z, but have differing amounts of neutrons, N.
Discrete Energy and Radioactivity
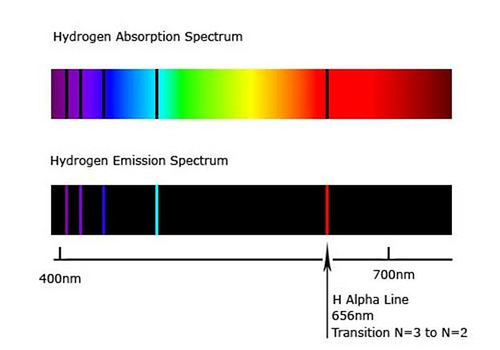
Electrons of an atom can occupy certain discrete atomic energy levels. As an electron makes a jump from one energy level to another, energy is absorbed or released in the form of a photon. The amount of energy absorbed or released is equal to the difference between the discrete atomic energy levels and is also quantized. The energy of a photon is dependent on its frequency. Therefore, only photons with frequencies which correspond to the differences between the atomic energy levels can be absorbed or released by an atom. These frequencies appear as spectral lines in the emission and absorption spectra.
Emission and Absorption
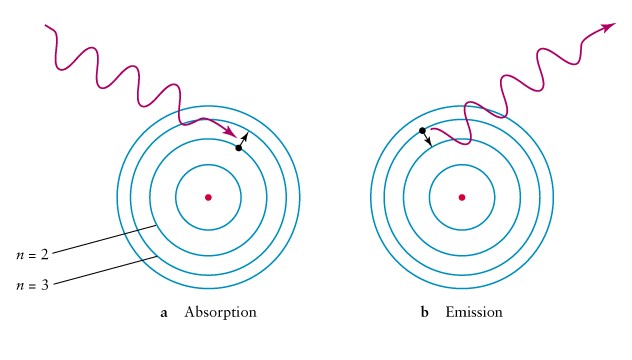
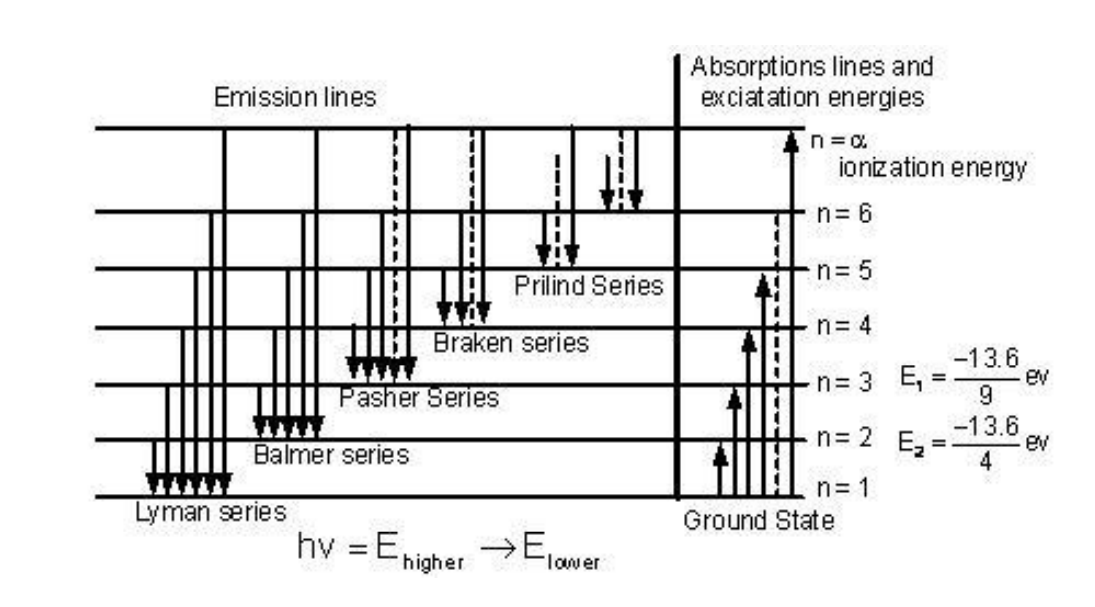
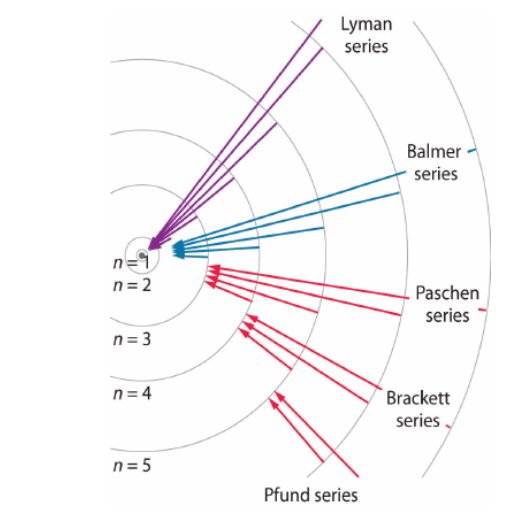
The existence of discrete energy levels called atomic energy levels can be supported by the emission spectra and the absorption spectra of atoms.
Transition Between Energy Levels
When the electrons within an atom jump from one atomic energy level to a lower energy level, energy is released in the form of light. Likewise, light is absorbed when the electrons within an atom jump from one atomic energy level to a higher energy level.
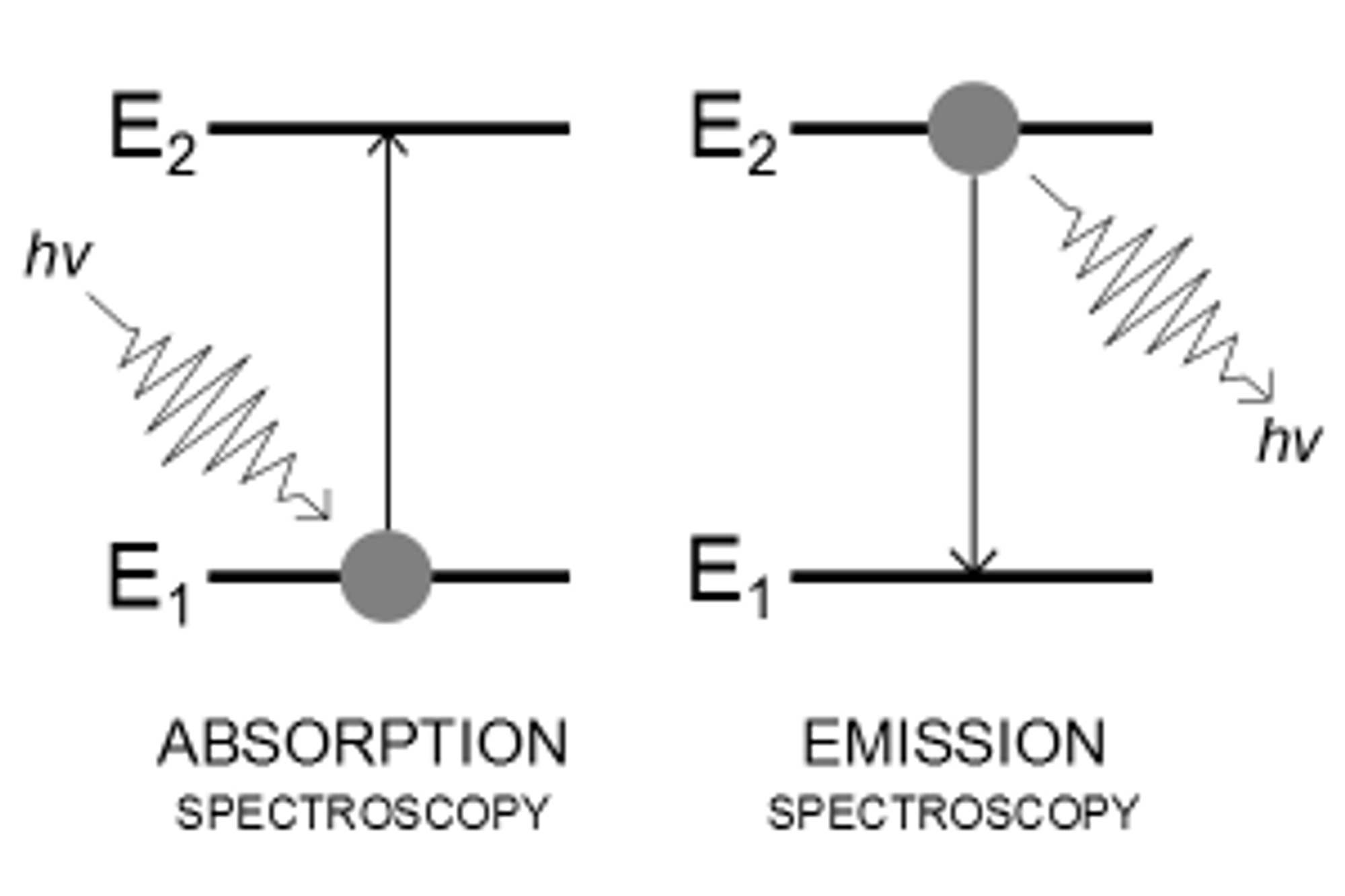
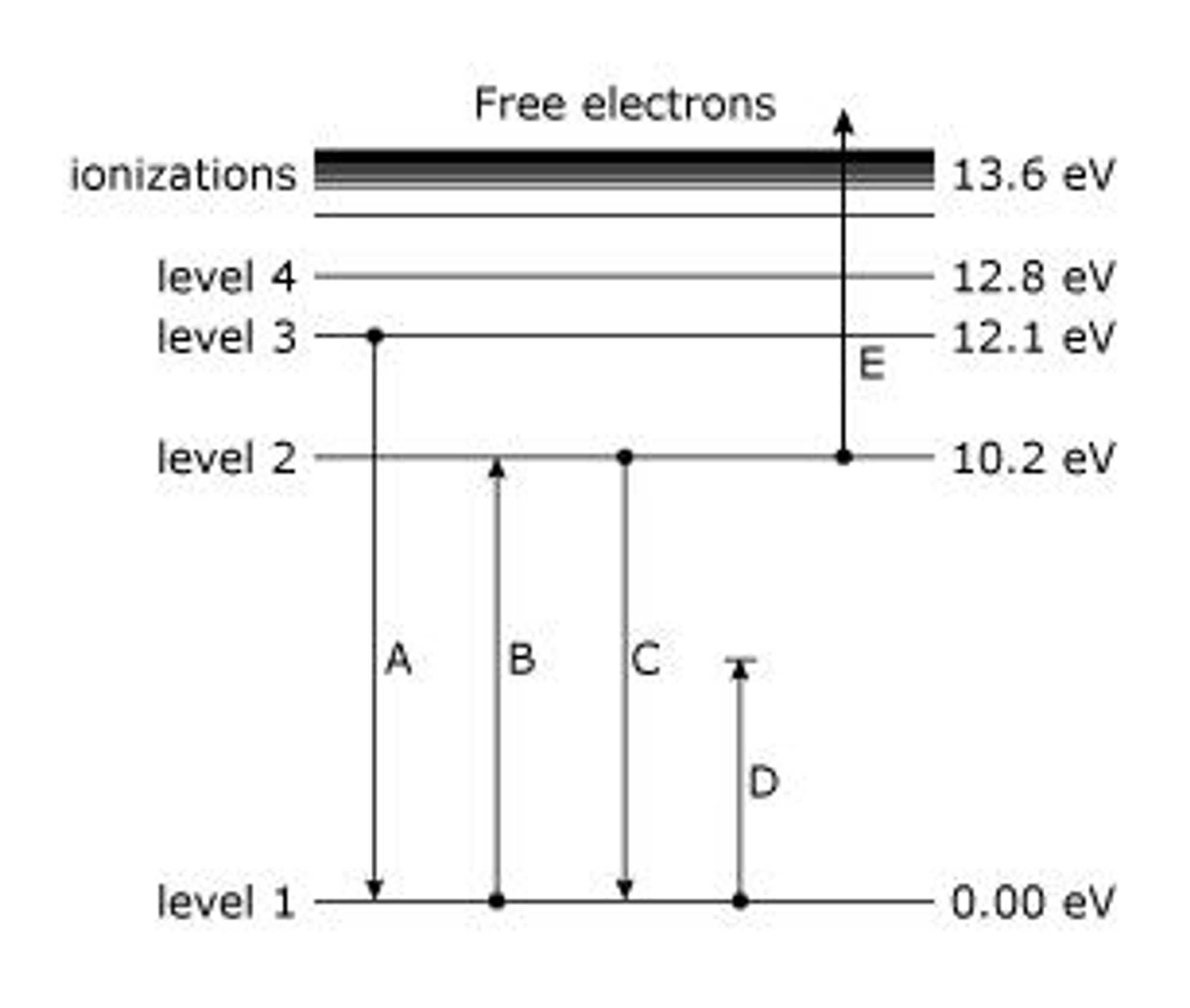
The amount of energy absorbed or released can be calculated by the difference in energy (eV) between the two energy levels.
Radioactive Decay/Half Life
Radioactivity was discovered by accident in 1896 by Henri Becquerel. He was studying fluorescence of materials when they were placed near X-Ray and Cathode Ray tubes. Some materials would even fluoresce when exposed to sunlight (phosphorescent). It was proposed that he reverse might be true, that phosphorescent chemicals could emit weak X-Rays when exposed to sunlight.
One day a sample containing uranium was tested on a dark cloudy day. He put the plate holding the sample in a drawer. Later when he developed the plate he expected a weak exposure but what was seen was a strong exposure. His only conclusion was that the uranium compound emitted some radiation which penetrated the paper exposing the film. Becquerel had discovered radioactivity.
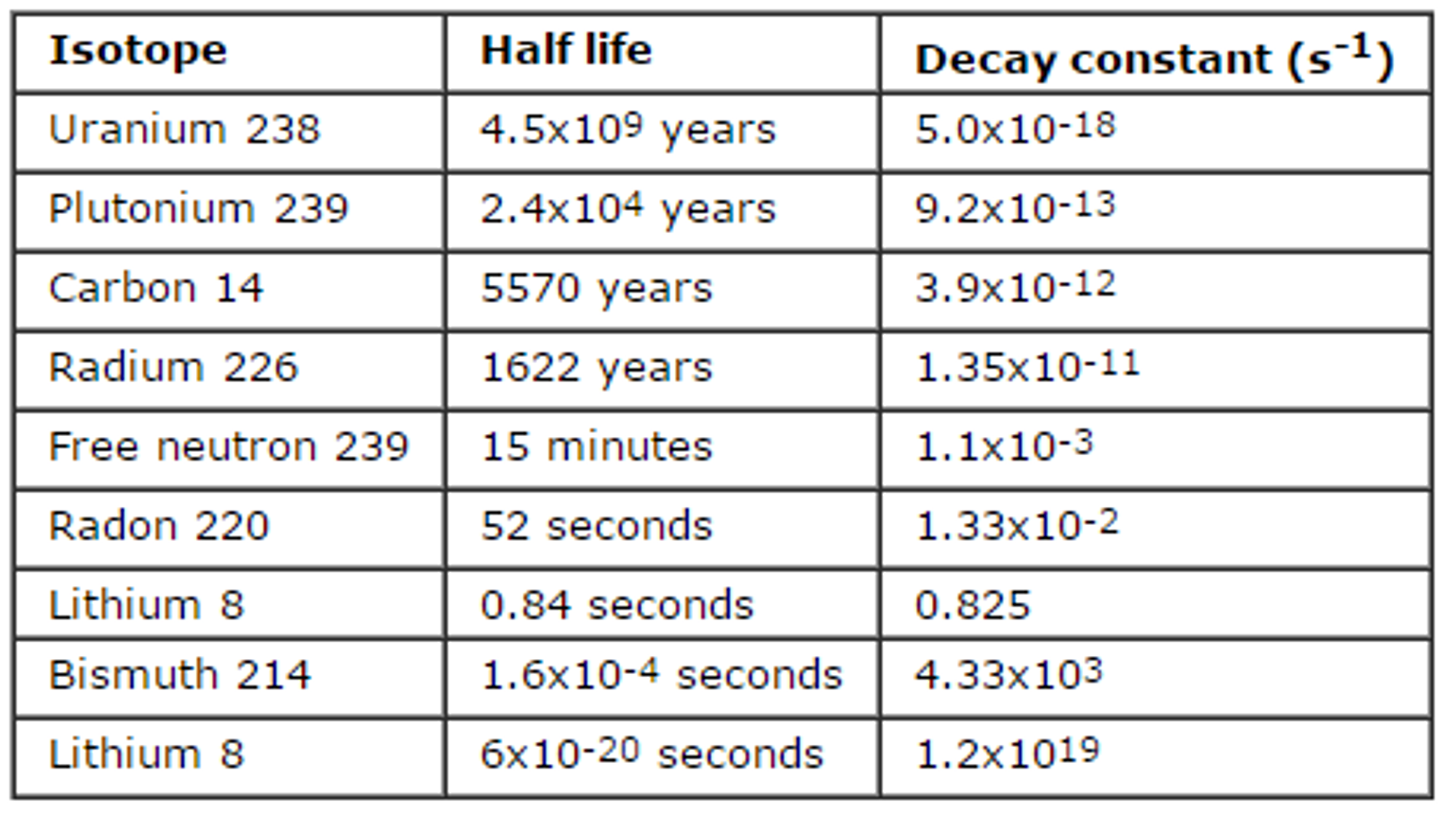
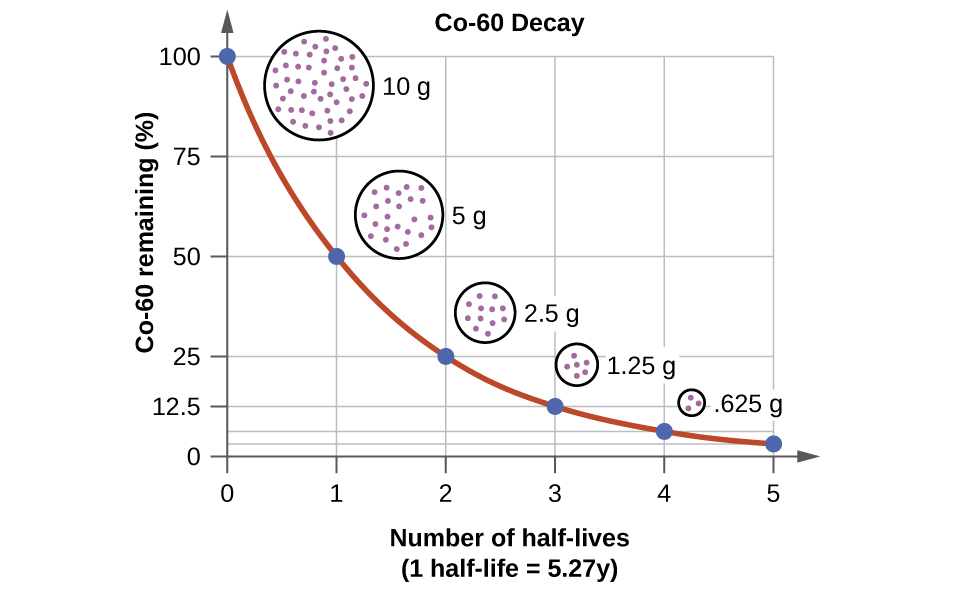
Radioactive decay refers to the spontaneous random process by which particles or electromagnetic radiation is emitted from an unstable nucleus. The product nucleus from a radioactive decay is called a daughter nucleus. The daughter nucleus is energetically unstable. The activity of radioactive decay can be shown by half-lives. This formula expresses activity (half-life decay), (decay/s) to be equal to some base rate, that takes , the half life (s), to reach 1/2 of the original amount. ( is the time elapsed and often measured). This formula can be manipulated into the following formula for half-life: The radioactive half-life of a substance is the time it takes for half of its radioactive nuclei to decay. If we plot the amount of radioactive nuclei which have not yet decayed with time, the resulting curve is called the decay curve and can be shown as the following.
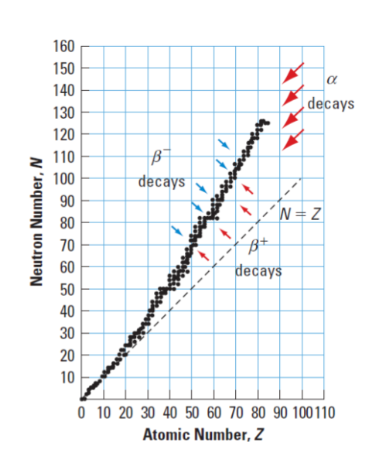
Alpha () Decay
Alpha decay typically occurs in heavier nuclei (about 83 protons) but theoretically it can occur in elements heavier than nickel as the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spontaneous fission-type processes.
- An alpha particle is a helium nucleus.
- It has a relative charge of +2
- Its penetration power is the lowest among the three types of particles and can be blocked by a piece of paper or a few cm of air.
- Its ionizing power is the highest among the three types of particles.
The speed at which the alpha particle is ejected can be calculated using the following equation: (discussed later).
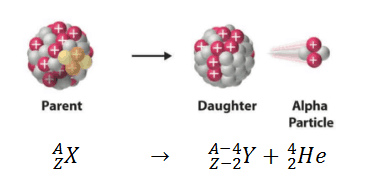
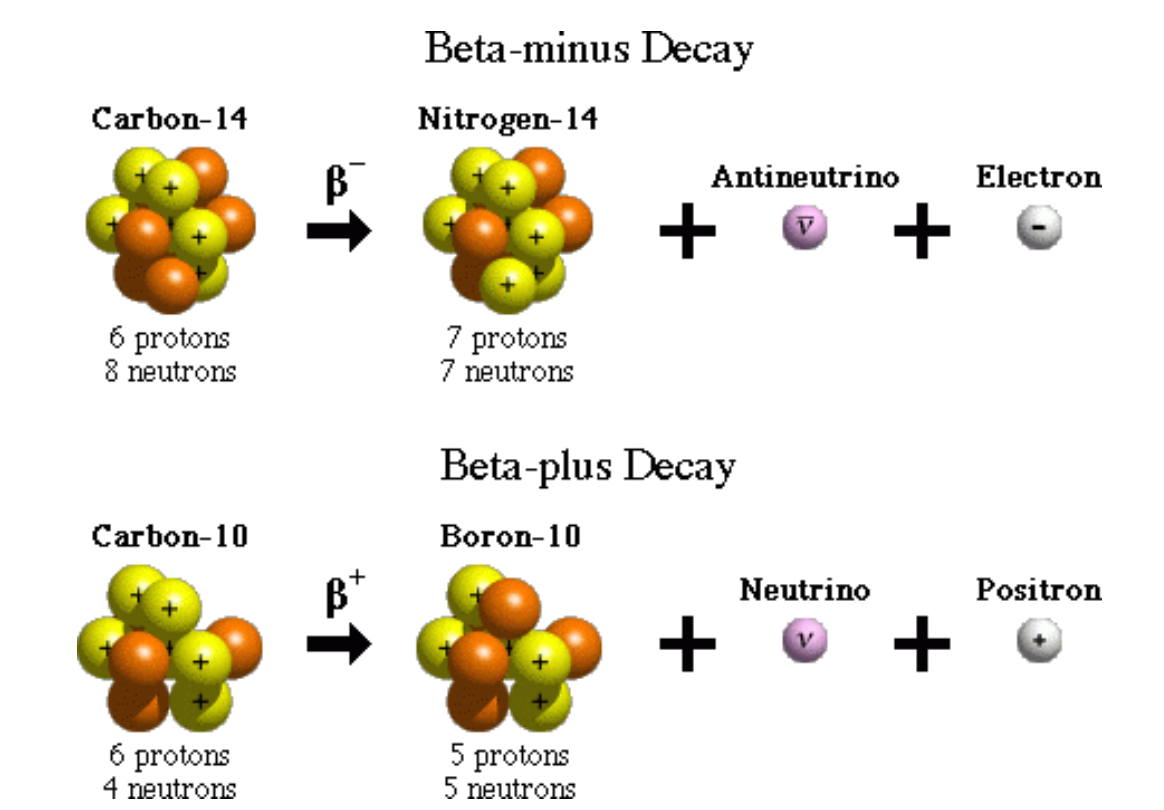
Beta - ( ) Decay & Beta + () Decay
Beta- decay occurs when the ratio of neutrons to protons is too high : an excess neutron transforms into a proton and an electron. The proton stays in the nucleus and the electron is ejected energetically with an electron antineutrino.
Beta + decay occurs when there are more protons than neutrons ; causing a proton to turn into a neutron and a positron with a neutrino
Gamma Decay
After nuclei has undergone alpha or beta () decay, it is left with too much energy (in an excited) state. As gamma rays are photons, it does not change the charge. Its penetration power is the highest among the three types of particles and can be blocked by several cm of lead. Its ionizing power is in the lowest among the three types of particles.
Decay in Application
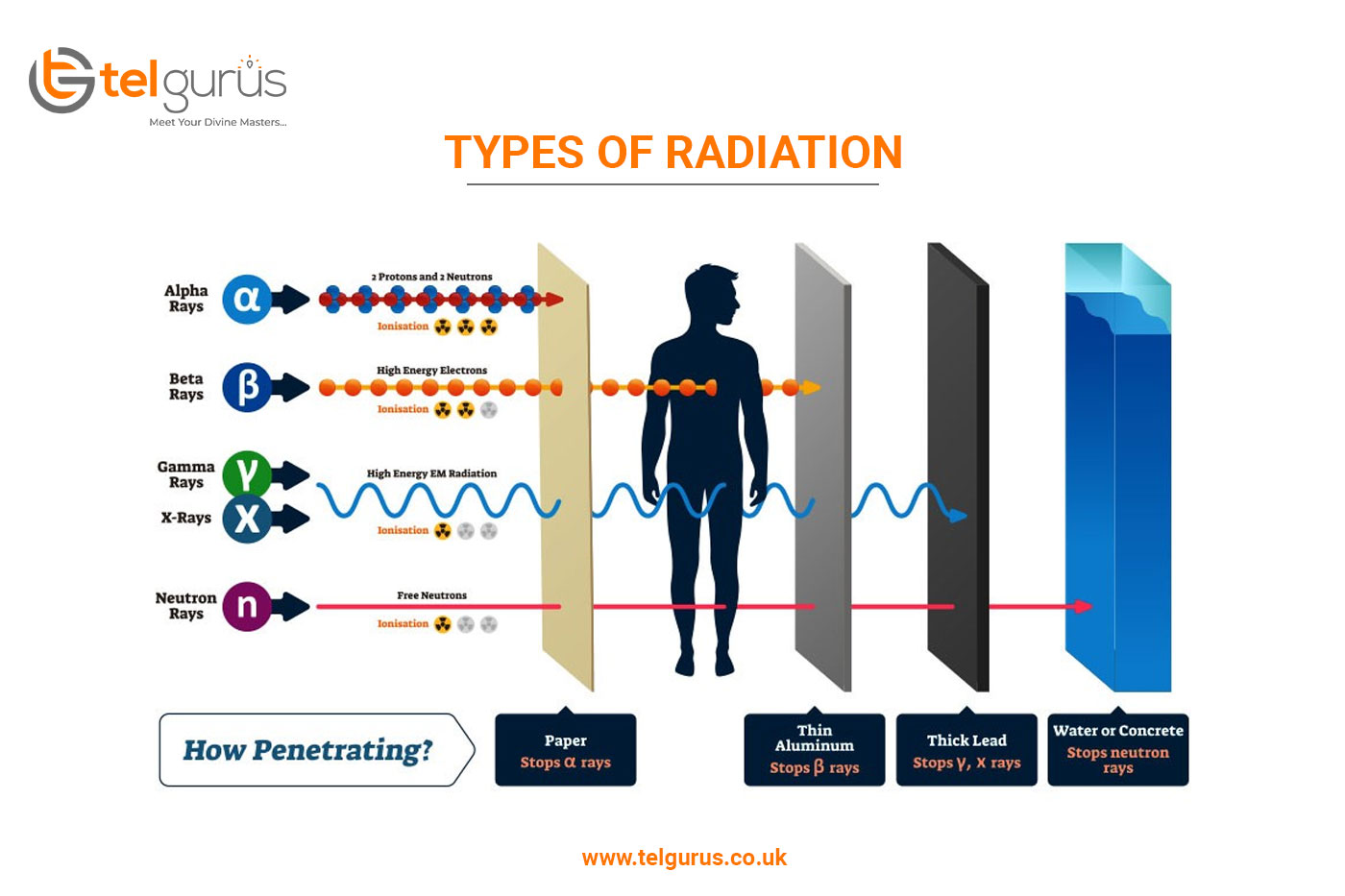
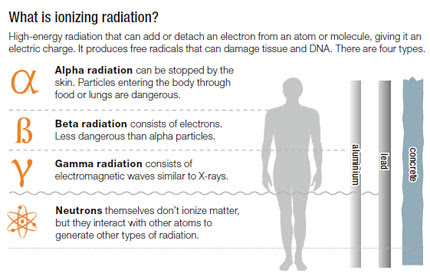
Background Radiation
Background radiation comes from natural sources and artificial sources.
- Natural sources: cosmic rays from space, radioactive rocks and soil, living organisms that have consumed radioactive substances in the food chain
- Artificial sources: radioactive waste from nuclear power plants, radioactive fallout from nuclear weapons, medical x-rays Average composition and exposure of background radiation
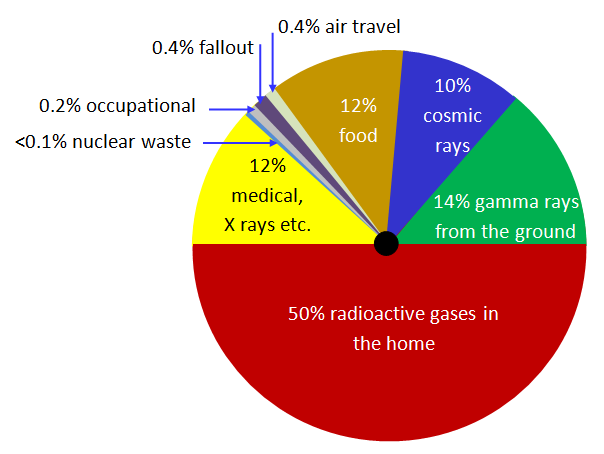

Chain Decay
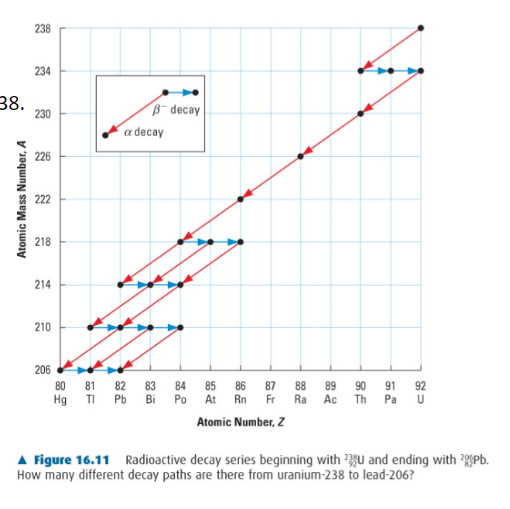
When a substance decays, if often decays into another highly radioactive substance, which is also highly likely to decay further.
Emission
Antimatter
Beta decay involves antimatter; an antimatter particle has a key property, such as charge to that of the corresponding particle of ordinary matter. For example an anti-electron or a positron or has a positive charge but the same mass of an electron. If matter is created, antimatter is also created (pair production)
Neutrino
Physicists expected that virtually all the energy released during decay would appear as the kinetic energy of the electron emitted by the nucleus. However, measurements found that most electrons emitted during decay had somewhat less kinetic energy than expected, and a few had almost no kinetic energy. Wolfgang Pauli suggested a very small neutral yet undiscovered particle was taking away missing energy. This is the neutrino; there are two types.
In decay an antineutrino, is released. In is a neutrino is released. The neutrino and antineutrino are identical in all aspects excluding their spin. Anti particles are commonly indicated by adding a bar over the symbol for the corresponding normal matter. For example an antineutron is represented by the symbol, .
In the beta transformation of a neutron into a proton, it involved a fundamental weak force that acts on electrons and neutrinos, whereas strong nuclear force does not.
- A neutrino is a type of lepton. Since they have no electrical charge or strong charge, most neutrinos do not react with other particles and pass right through earth with no interaction.
- Neutrinos are produced in many particle decays, such as in beta decay. When a neutron at rest (zero momentum) decays by releasing a proton and an electron, because of the law of conservation of momentum, the resultant products of decay must have a total momentum of zero, which the observed proton and electron clearly does not portray. Therefore, we suggest the presence of another particle to balance the momentum – by the release of an antineutrino (neutrino antimatter). This was confirmed by experimentation.
- Neutrinos were produced in great abundance in the early universe and rarely interact with matter. This may suggest that neutrinos contribute to the total mass of the universe and affects its expansion.
Detecting and Measuring Subatomic Particles:
- Geiger counters record the energy of particles or photons striking the detector as a surge of V
- Cloud chambers contain dust-free air supersaturated with vapour from a liquid such as water or ethanol. A charged particle speeding though the supersaturated air will ionize some molecules along its path, triggering condensation.
- Bubble chambers contain a liquified gas such as hydrogen, helium, propane or xenon. When the pressure is reduced so that the boiling point is just below the actual temperature of the liquid, ions formed by the charged particle moving through the liquid cause boiling, thus the particle forms a trail of tiny bubbles along its path.
- ** When answering long answer questions on nuclear radiation, you need a method to observe the radiation, such as a cloud or a bubble chamber. The radiation is not visible without such instruments.
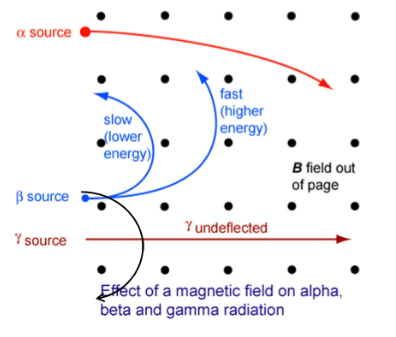
Magnetic Fields and Charged Particles
Magnetic fields are typically used to bend the charged particles into a circular path (Centripetal motion.) So for a particular magnetic field the radius of the path depends on the particle, the speed and the charge. The more massive alpha particles tend to bend the least as they have significantly more mass than beta particles; therefore the radius bend is much larger.
(This has no effect on gamma radiation as it has no charged particles)
Electron Capture
This is when a high speed electron is captured by the nucleus changing a proton to a neutron. This results in the emission of an electron into a neutrino.
Nuclear Reactions
When discussing nuclear reactions, the unified atomic mass unit (μ) is commonly used in nuclear physics. It is defined as one twelfth of the mass of a carbon-12 atom. This is particularly useful in finding the difference in mass of a nuclear reaction (to account for speed of a particle ejected for example)
Mass Defect:
The difference between the mass of an atom and the sum of mass of its constituent parts is called its mass defect. Mass defect can be explained by Einstein’s mass-energy equivalence: As energy required to break apart a nucleus, the sum of energy contained in the constituent nucleons is higher than that of the combined nucleus. Energy is related to mass.
This was based on the principle that a certain amount of energy has an equivalent mass. Physicists interpret the mass defect as the binding energy that holds the protons and neutrons together in the nucleus. Due to the large forces between protons, a large amount of energy and large forces are required to hold the nucleus together. The binding energy results from a strong nuclear forces.
The nuclear binding energy of a nucleus is the amount of work required to separate the nucleons inside the nucleus. Binding energy per nucleon = binding energy of nucleus / number of nucleons in nucleus. This is where Einstein’s equation relates the rest mass to an equivalent energy If then of energy (where is the speed of light)
Nuclear Binding Energy
The nuclear binding energy is the minimum energy required to disassemble the nucleus of an atom. As nucleons are attracted to each other by the strong nuclear force, the difference in mass when a nucleus undergoes nuclear decay is equal to the mass defect. (Energy may still be lost to kinetic energy of particles).
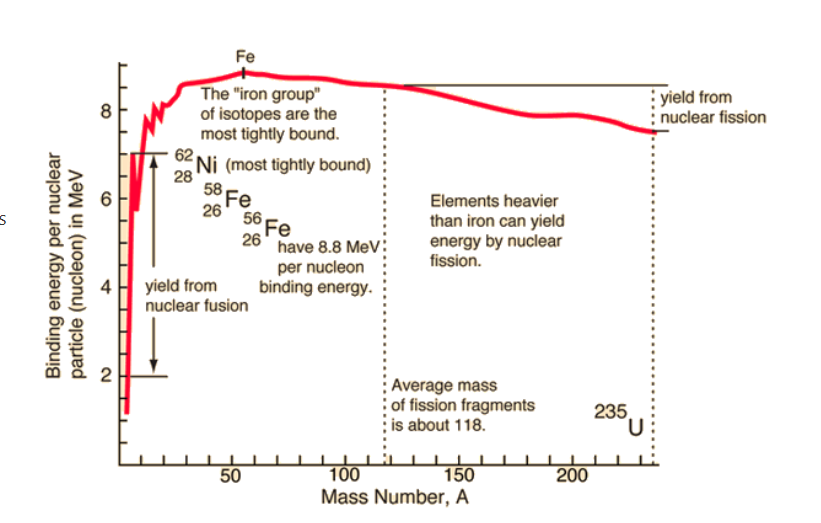
Nuclear Fission
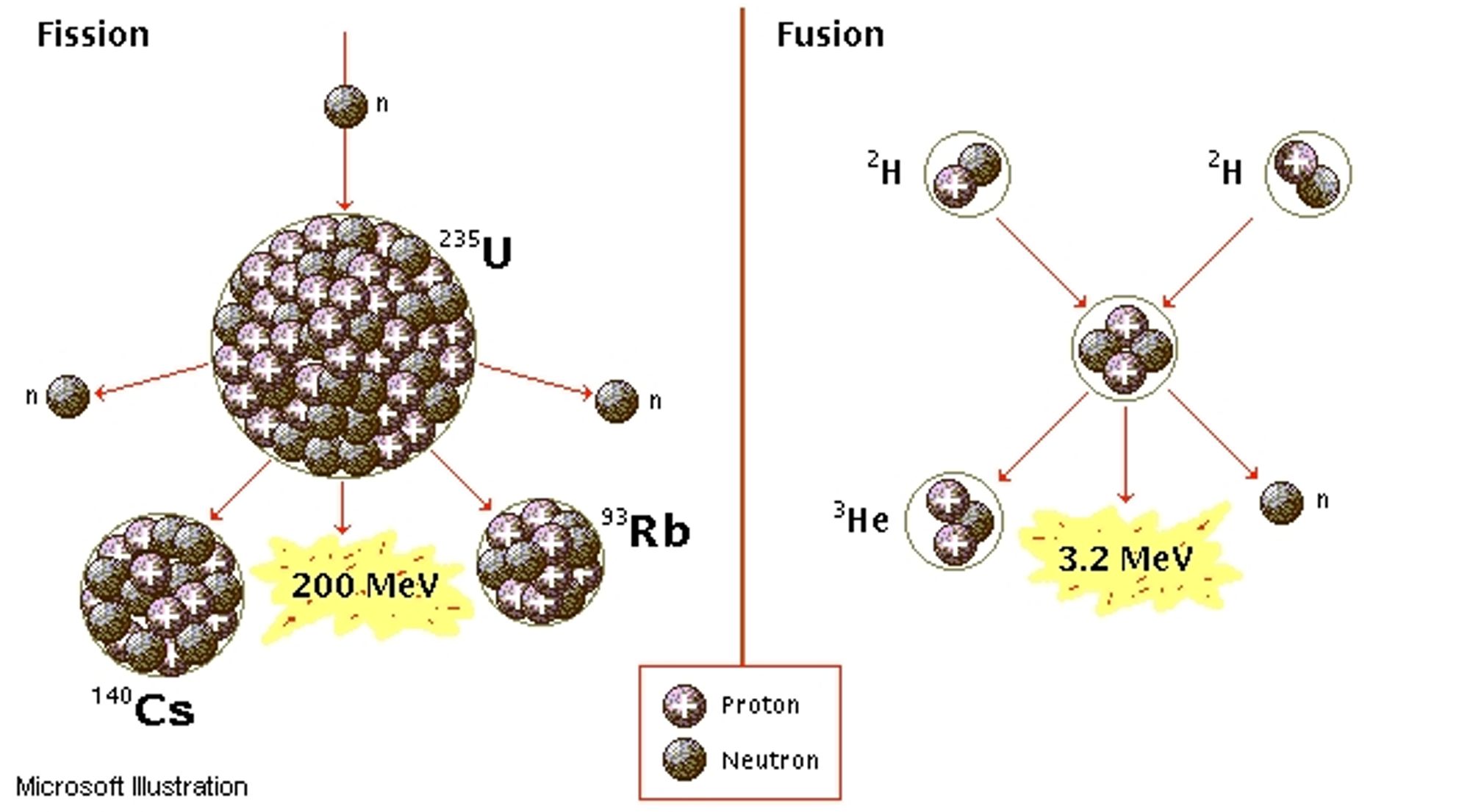
Nuclear fission is the splitting of a heavy nucleus into two lighter nuclei. A great amount of energy is released in nuclear fission due to the greater binding energy of the daughter nuclei. Nuclear fission is used in nuclear power plants. When a nucleus with an atomic number above 120 splits into smaller nuclei, each product nucleus has greater binding energy per nucleon than the original nucleus. Additionally, the mass of reactants is greater than the products formed;
Problems: Highly radioactive by-products (waste); nuclear meltdowns; fuel and byproduct can be used to make bombs. Benefits: High E/kg of fuel used. No emissions. For application of the energy produced in these nuclear reactions see Energy production
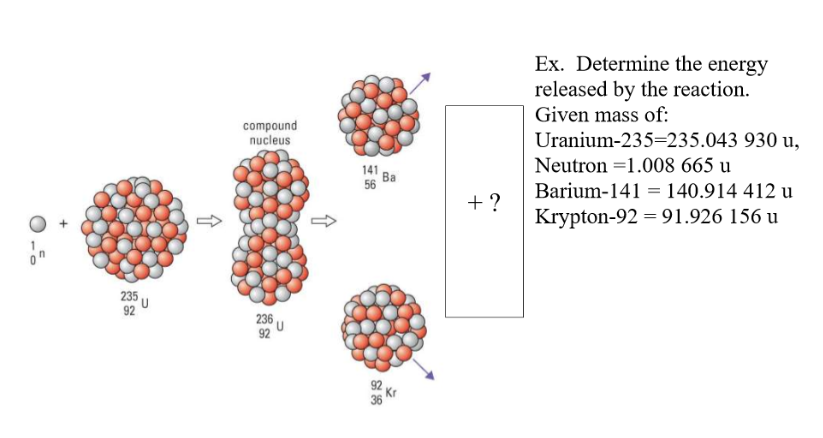
Nuclear Fusion
When two low-mass nuclei combine to form a single nucleus with an atomic mass less than 60, the resulting is more tightly bound. This fusion reaction gives off energy equal to the difference between the total binding energy of the original nuclei and the binding energy of the product. Additionally, the total mass before the reaction is greater than than the total mass after the reaction. There fore the energy released is
Problems: Requires huge amounts of energy to overcome electric force opposing nuclei from fusing together. This makes a sustained controllable reaction impossible.
Benefits: Requires more energy released per mass of fuel used. There is an abundance of easily obtained fuel. Zero emissions. There are no radioactive by products.
- Nuclear fusion is the joining of two light nuclei to form a heavy nucleus.
- A great amount of energy is released in nuclear fusion due to the greater binding energy of the daughter nucleus.
- Nuclear fusion yields more energy than nuclear fission.
- Nuclear fusion is the main source of the sun’s energy.
Interactions with Light
Photoelectric Effect
Einstein proposed that light consists of particles called photons. Quantum refers to the smallest discrete amount of something. A photon is a quantum of electromagnetic radiation (light). Photons exhibit wave properties under refraction or interference. Photons exhibit wave properties under its emission or absorption.
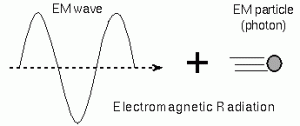
Where a photon’s energy, is given by: where is Planck’s constant, is the speed of light, and λ is its wavelength (electromagnetic wave).
Given this property, the photoelectric effect can be better understood: The photoelectric effect refers to the emission of electrons from a metal surface as a result of the absorption of electromagnetic wave energy.
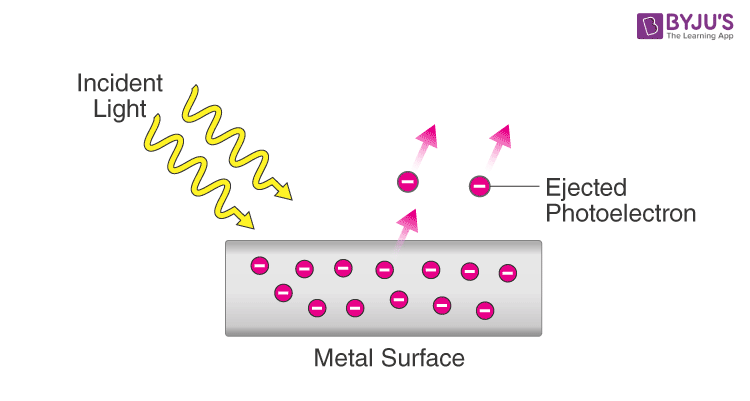
The intensity of the light affects the number of electrons ejected, where the frequency of the light determines the kinetic energy of the ejected electrons. There exists a minimum, threshold frequency that must be reached before this effect is observed.
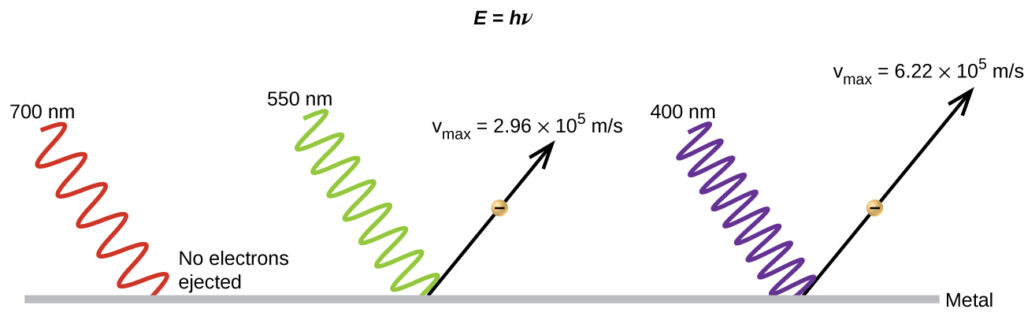
Incident electromagnetic waves with lower frequency have a smaller chance of inducing the photoelectric effect.
The number of photons per unit time in the incident light is proportional to the light intensity. An increase in the intensity of the incident light allows a higher number of photon-electron interactions. Therefore, more electrons are ejected.
There exists a minimum energy below which electrons would not be ejected from the metal. This minimum energy level depends on the metal in use and is called the work function (φ).
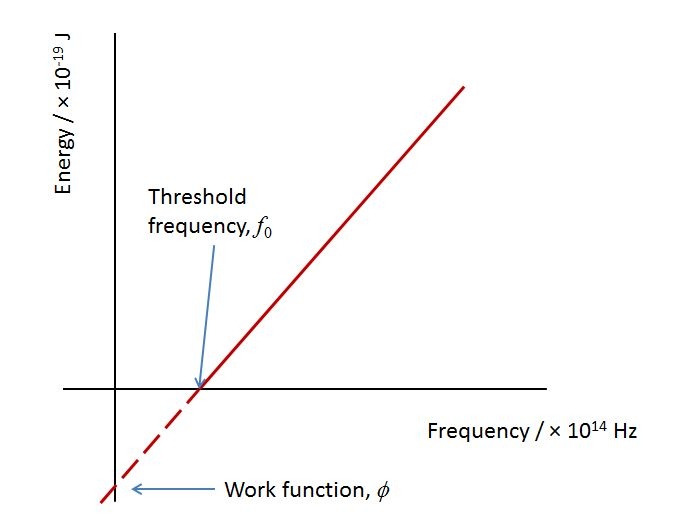
(Gradient = h, y-intercept = work function)
Since E=hf, φ=hf0 where f0 is called the threshold frequency. Threshold frequency - The minimum frequency of indecent radiation that will cause the ejection of a photoelectron from a photoelectric surface -
Planck’s constant - the constant of proportionality relating the energy of fa photo to its frequency
Stopping voltage - the minimum voltage required to stop the most energetic photoelectrons from complete a photoelectric circuit.
Compton Effect !!
X-rays and graphite - electrons were meter, with lower energy X-rays. E was conserved
Momentum is also conserved - vectors
= conservation of momentum and energy
Wave particle duality = the exhibition of both wave-like and particle properties by a single entity
Newton vs Huygens:
Newton supported particle, Huygens supported wave. Before photoelectric and wave of Maxwell was accepted as standard.
Young + his experiments with interference provided string evidence for the wave model.
Maxwell - transverse wave + prev properties.
Hertz + Einstein - particle model
Matter Waves
The De Broglie hypothesis suggests that all matter exhibits wave-like properties. In particular, the momentum of a particle is related to its wavelength where the De Broglie wavelength may be deduced by the following formula: Where is momentum, is Planck’s constant, λ is wavelength, is mass, and is velocity. The term “wave-particle duality” refers to matter acting as both waves and particles.
Pair Production and Pair Annihilation
All matters have their antimatter counterparts which resemble their corresponding matter in every way except for the sign of their charge and the direction of their spin.
Pair production = When a high energy photon collides with a nucleus, it makes a pair of electron and positron (electron antimatter) and gives kinetic energy to each particle.
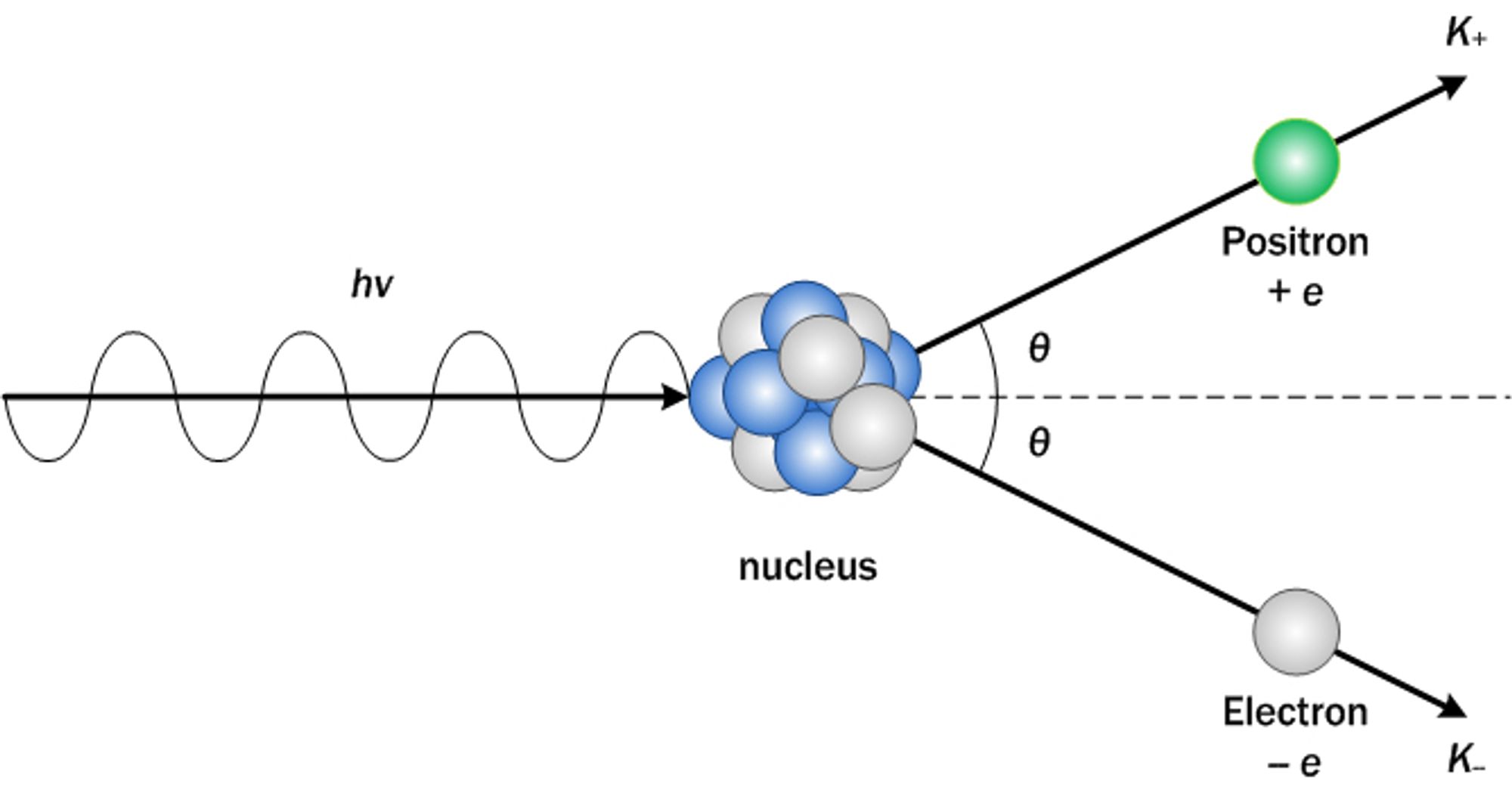
Pair annihilation = When matter collides with its corresponding antimatter, they annihilate one another with the conservation of energy, momentum, and charge.
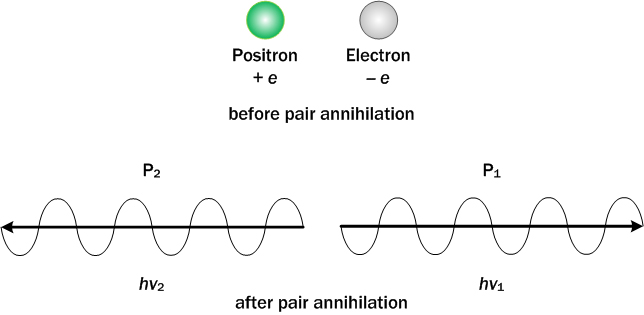
The positron (+e) collides with the electron (-e), annihilating each other into two photons with exactly opposite directions and the same amount of momentum.
Quantization of Angular Momentum for H (Bohr Model)
Bohr developed a model for hydrogen that was able to explain the emission and absorption spectra of hydrogen. His model assumed discrete orbital paths in which electrons orbit the nucleus through, the same way planets orbit stars. The orbits were quantized in terms of their allowable angular momentum (rotational momentum). Therefore, the orbital radii and energies are also quantized. The energy of the orbit is the energy required to ionize (remove) an electron and can be given through the following equation in relation to the order of orbit (n)
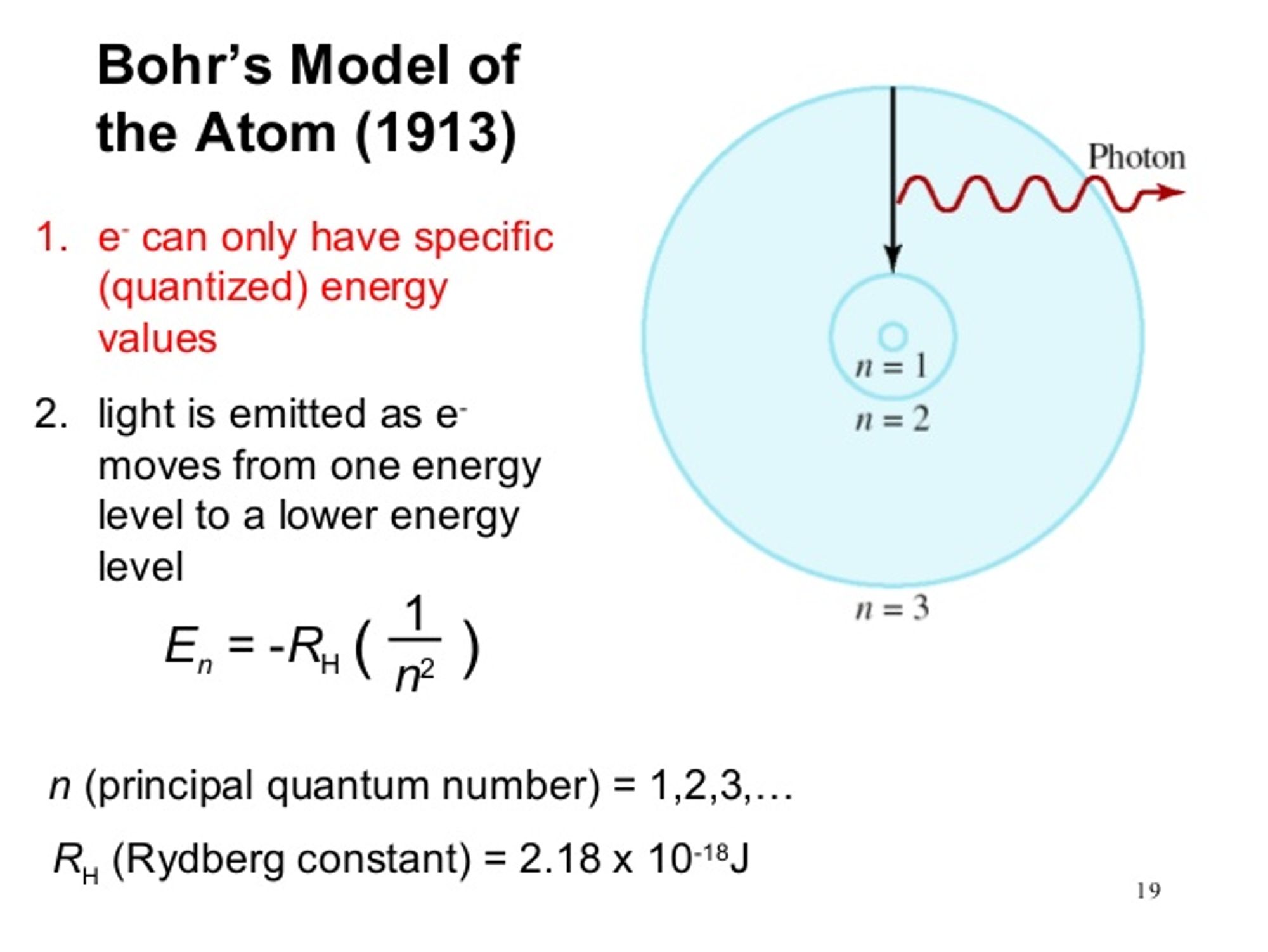
When the electrons are excited, they jump to higher energy orbits and eventually drop back down to a more stable orbit by releasing excess energy by the form of light. The energy of the light released is therefore equal to the difference in energy of the two orbits.
Wave Function
By quantum physics, all particles do not have a defined position until they are observed. Instead, all particles are described as “a wave function.” Each “particle” is represented by a wavefunction (position, time). When applied in the Schrödinger equation (based on Newtonian laws and the conservation of energy in classical mechanics) predicts the future behavior of a dynamic system. It predicts analytically and precisely the probability of events or outcomes.
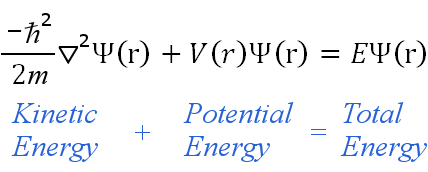
- Contains all the measurable information about the particle.
- summed over all space = 1 (if the particle exists then the probability of finding it must be 100%)
- is continuous
- allows energy calculations via the Schrödinger equation
- establishes the probability distribution in three dimensions
- Permits calculation of the effective average value (expectation value) of a given variable
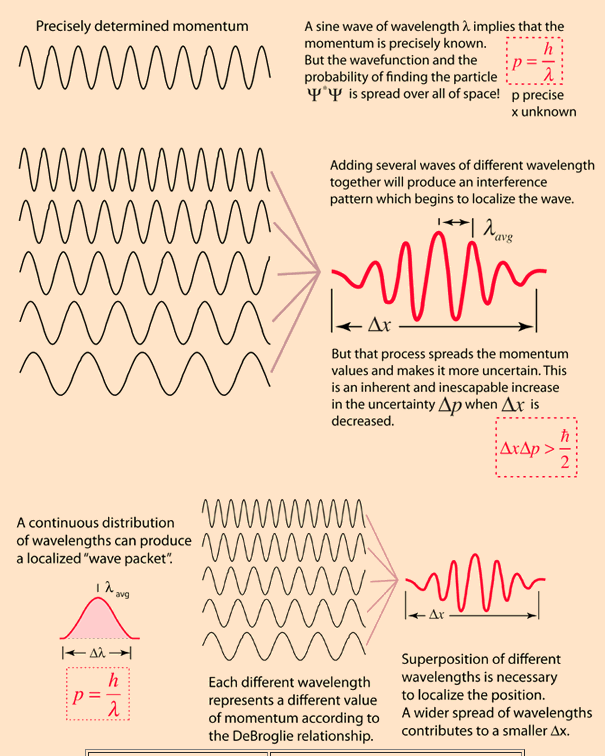
- For a free particle is a sine wave, implying a precisely determined momentum and a totally uncertain position (uncertainty principle)
Uncertainty Principle for and ??
The Heisenberg uncertainty principle states that: If the energy state only lasts for a brief period of time, its energy is uncertain.Position and momentum cannot be measured simultaneously with precision. The more precisely the position is determined, the less precisely the momentum is known, and vice versa.’
Tunnelling & Potential Barriers ??
Imagine throwing a ball at a wall and having it disappear the instant before making contact and appearing on the other side. The wall remains intact and the ball did not break through it. Believe it or not, there is a finite (if extremely small) probability that this even would occur. This phenomenon is called quantum tunnelling.
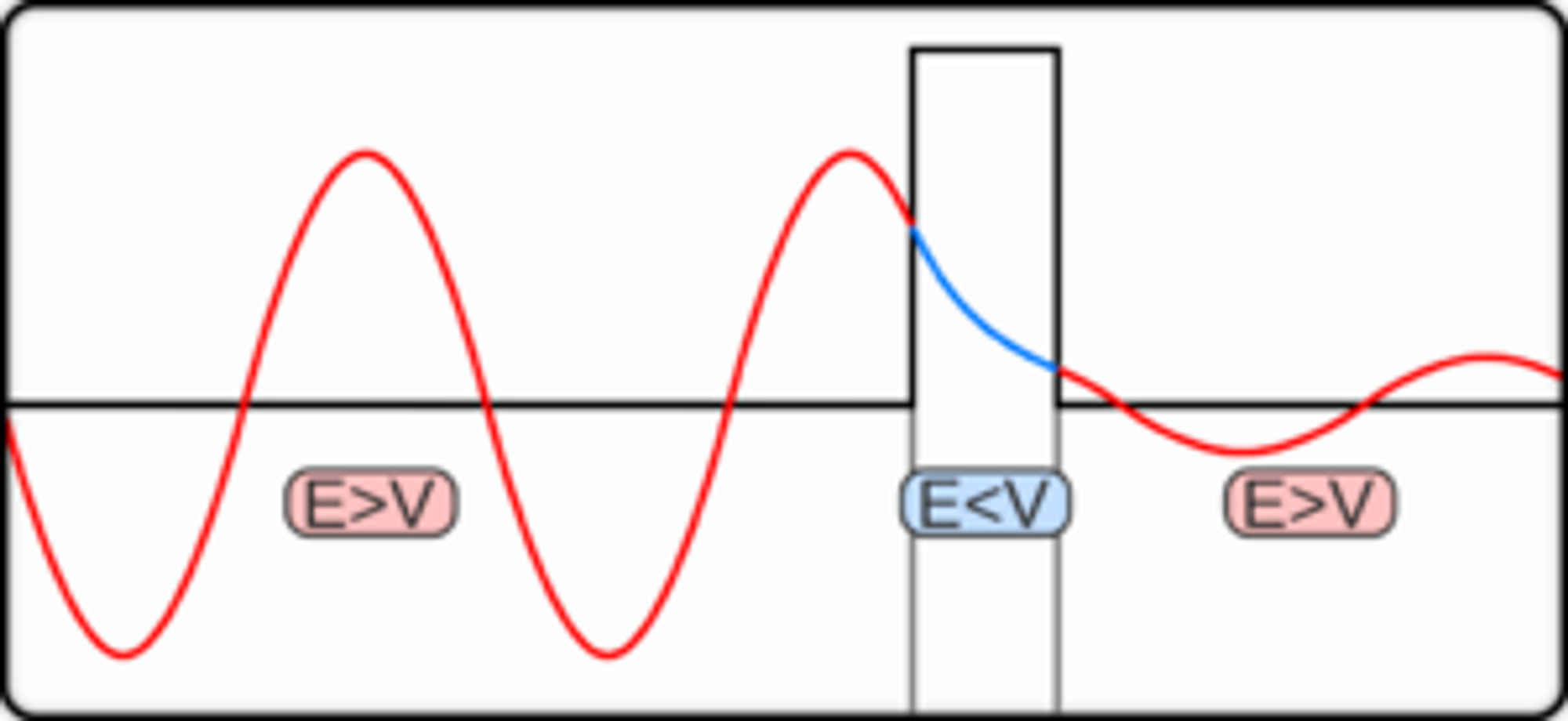
The position of a particle is described as a wave function (see previous section)
From the graph above, the observable particle is most likely to be at the position where its wave function has the largest amplitude. However, although the amplitude of the wave function will decay exponentially, since the wave function does not reach an amplitude of zero, the wave function can exit the barrier. Once the wave function exits the barrier, its amplitude no longer decays. This means that a particle has a certain probability of bouncing off a barrier and a certain probability of passing through the other side.
| Factor | Effect towards tunnelling probability |
|---|---|
| Increase barrier length | Decrease |
| Increase particle mass | Decrease |
| This explains how tunnelling is frequent in nanoscale but negligible at the macroscopic level. |