2024-07-1113:41 Status:IBnotes
Introduction
Organic compounds are compounds that contain carbon, excluding oxides of carbon, , carbonates, carbines and cyanides. Organic compounds cover a large portion of known chemicals - of the 4 million known chemicals, 40,000-50,000 chemicals are inorganic. This includes lipids, carbohydrates, proteins, fuels, fabrics, medication, plastics, soaps, detergents, fertilizers.
Hydrocarbons
Compounds that are formed strictly from carbon and hydrogen. These are covalent compounds and have two sub-categories: aliphatic and aromatic.
Derivatives of Hydrocarbons
Aliphatic
These are basic hydrocarbons that contain only single bonds between carbons and hydrogen atoms. Between carbon and carbon atoms, single bonds are labelled as alkanes, double bonds are labelled as alkenes, and triple bonds are labelled as alkynes.
Representation of Organic Formulas
Molecular Formula
This can be expresses as the total amount of each element (). The empirical formula is the lowest ratio of elements( ).
Expanded Structural Formula and Simplified Structural Diagram
Structural diagrams show all the atoms present in an organic molecule. This includes explicit denotation of carbon, hydrogen and other atoms. The following is the expanded structural formula for . (Omitting hydrogen is the simplified diagram)
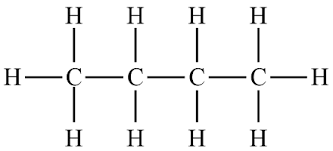
Condensed structural formulas/condensed formula
cC_4H_{10}$$CH_3-CH_2-CH_2-CH_3 (where are collapsed, and carbon bonds are shown) or (Where each carbon-hydrogen bond is grouped together and carbon-carbon bonds are omitted).
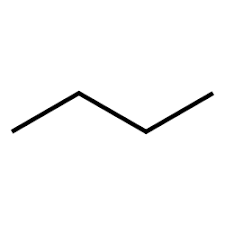
Skeletal Structural Formula/Bond-Line Diagram
In these diagrams, only carbon-carbon bonds are shown. Additionally, the nodes that connect two edges (or terminate an edge) are where carbon exists. Hydrogen is assumed to be bonded with the remaining valance electrons of each carbon atom. Chains of carbon are typically drawn in the following ‘zigzag’ shape ( ):
This is the most common way that organic molecules are drawn.
Alkanes
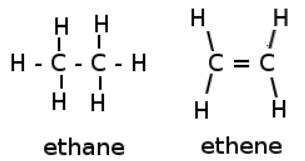
The general formula for alkanes are . They are saturated hydrocarbons, they have the maximum amount of hydrogens attached. This is because these molecules only have single bonds. Thus, the remaining spots for binding are open for (primarily) hydrogen to bond to.
Alkanes are also non-polar as they are being ‘pulled’ in directions that cancel each other out.
Some examples of alkanes include: methane , ethane , propane , butane .
Branching
If a hydrogen atom is taken off of an alkane, it turns into a branch denoted with a replaced ending of -yl: Methane → methyl, ethane → ethyl → propane → propyl, butane → butyl.
Nomenclature
- Determine the longest chain of carbon.
- The longest chain becomes the parent chain/main chain
- Give this a proper alkane name: Methane (1), ethane (2), propane (3), butane (4), pentane (5), hexane (6), heptane (7), octane (8), nonane (9), decane (10)
- Number each of the carbon in the parent chain. This allows addressing for branches.
- Name the attachments (alkyl groups) and identify their location.
- If the same branch exists at multiple addresses, add the prefix di-, tri-, etc.
- Add comma separated ascending addresses for the positions.
- Write the groups alphabetically in front of the parent name (with their address)
Example: 4-ethyl-2,3-dimethylhexane
- Draw out a strand of 6 carbon
- Attach methyl (1 carbon branch) to carbons 2 and 3
- Add ethyl (2 carbon branches) to the carbon at position 4
- Once all the carbons have been attached, add as many hydrogen atoms as possible.
- The resulting chemical formula for this is: a. Wherein .

Homologous Series
A series of molecules in the same group/family that only differ by a repeating unit (usually ) between each member. They have the same general formula. They have the same general formula For instance: methane , ethane , propane , butane are a homologous series.
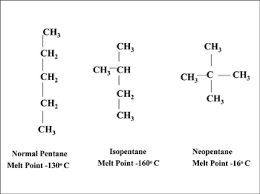
Isomers
These are compounds that have the same molecular formula but have a different structure and different name. In general the amount of isomer for alkanes, are smaller than that of alkenes, and alkynes as the presence of only single bonds limits the possible permutation of isomers. All the isomers for include:
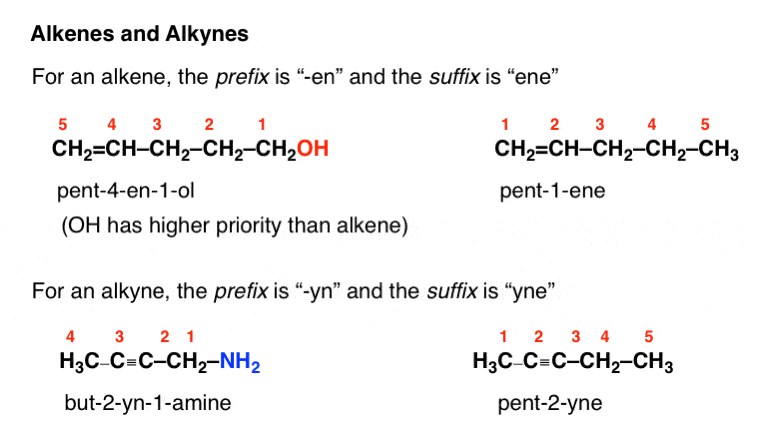
Alkenes
Alkynes, alkenes, and cyclics are all unsaturated. As removing a pair of results in the formation of extra carbon-carbon bonds. From alkenes to alkynes (, the more hydrogen pairs that are removed, the more unsaturated it becomes. This can theoretically go on forever, but those circumstances become increasingly unlikely.
The general formula for alkenes are if there is only one double bond present. The parent chain must contain a double bond, even if that shortens the chain. The double bond has the smallest possible address. (In chemistry 30 - alkene branches are not used.)
Naming
Depending on the position of the double bond, the name of the molecule changes. The old names denote the address of the double bond before the chemical name, however, it is the standard to now write the address of the double bond in between the prefix and the suffix. Additionally the ‘starting carbon’ is the lower of the two addresses and is used.
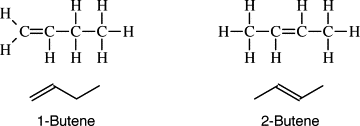
If butene had a double bond at the first carbon, it would be written as: but-1-ene.
Alkynes
These contain at least one triple bond. The general formula is and chains end in -yne.
Bromide Test
The Bromide test is used to detect alkynes and alkenes. Bromine water, having an orange/brown color attaches onto the double and triple bond. Thereby becoming transparent.
Cyclic molecules
Cyclic structures are ring structures that have a closed loop of carbon atoms. The prefix ‘cyclo’ is used to to the aliphatic parent name. In Cycloalkanes, cyclo- is the prefix.
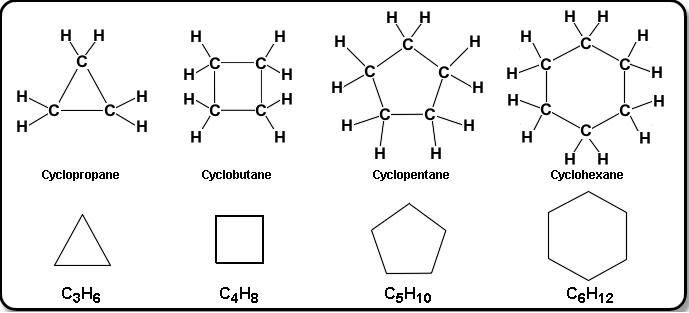
Naming
- The parent name is cycloalkane
- Numbering the carbon can start at any address; going clockwise or counterclockwise.
- If there are branches, give it the lowest consecutive numbers.
- Sometimes to break a tie, look to the other addresses (the winner will be found at the first lowest point of difference - regardless of sum)
- 1,2,8 vs 1,3,4 - the first one wins as 2<3 despite 8>4, and 11> 8.

Cycloalkenes
The general formula for cycloalkenes is , and the alkene should always have a double bond between the first and second carbon.
Aromatics
These are a unique group of hydrocarbons that are derived from a common parent organic compound: Benzene, . This is a cyclic compound that is arranged with altering single and double bonds (3 of each). In application, the single/double bond is not fixed and can be considered to be constantly swapping between a single and double bond.
This is shown in benzene (2).
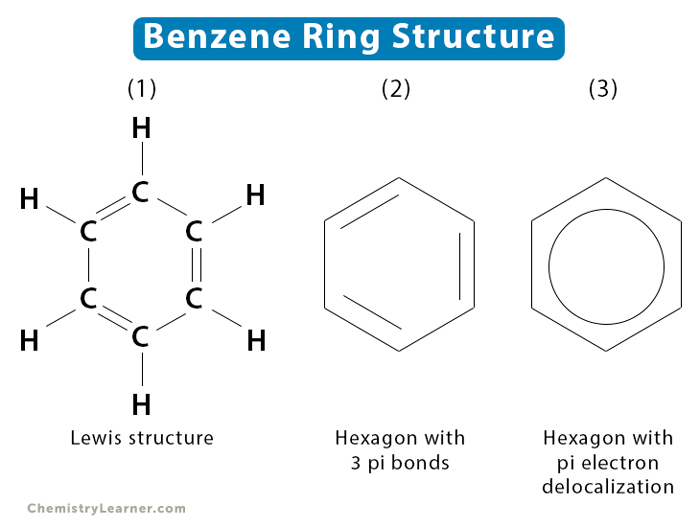
The classical way to represent a benzene molecule would be to denote single bonds with a singular line, and double bonds with two lines. However because a delocalized bond, the unhybridized p-orbitals in the otherwise structure from a resonance structure. This phenomena is denoted by replacing what would be a double bond with an arc between the single and double bonds. Thereby resulting in an a circular ring.
** In chemistry 30, aromatics are considered saturated.
Nomenclature
Benzene will be the parent name. Where in the name of branches follows the same rules as the cyclics. If a benzene is found in a branch, then it is replaced with the name ‘ phyenl-’ (benzene is not at the terminal end)
Physical Properties
- Colorless liquid at room temperature
- Has a sweet characteristic aroma at room temperature
- Non-polar compounds
- Evaporates very quickly (highly volatile)
- Breathing benzene can lead to dizziness, drowsiness, and unconsciousness.
- Long term exposure can lead to cancer
Benzene and its derivatives.
- Found in gasolines, dyes, plastics, pesticides, drugs, and medicines.
- Benzene can take on almost any attachment
- Aspirin
- Phenol-disinfectant.
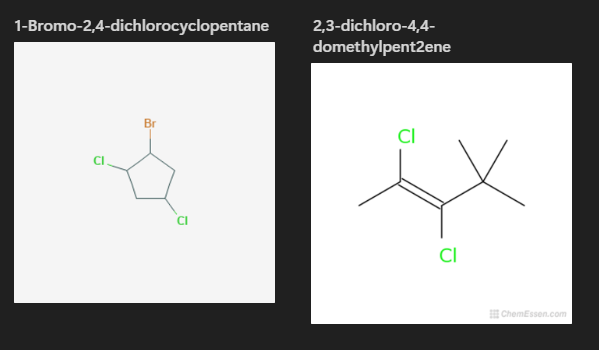
Halides
These are organic compounds that have one or more hydrogen branches replaced by a halide. These only exist as branches and they do not modify the parent name. In general, these compounds are toxic. Polarity and solubility both greatly vary.
Nomenclature
Like any other branch, the names communicate something specific about said branch. branch = flouro-, branch = chloro-, branch = bromo-, branch = iodo-.
Alcohols
The presence of a hydroxyl group gives alcohols its distinct characteristics. Alcohols have much higher melting and boiling points compared to compounds. Additionally they tend to have higher solubility in water (due to hydrogen bonding). All alcohols are toxic to human beings.
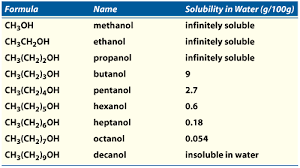
Nomenclature
Alcohol group takes priority, therefore must be in the parent chain. The endings change by dropping the -e and replacing it with -ol (indicating the location before the ‘-ol’).
It is also possible to have multiple alcohols, this is denoted by using di, tri, but do not drop the ‘e’.
If an is a branch it is because there is a ‘more important’ group in the chemical. Thus the alcohol then becomes a branch and is denoted with hydroxyl- (IOPAC prefers hydroxy-).
Classification of Alcohols
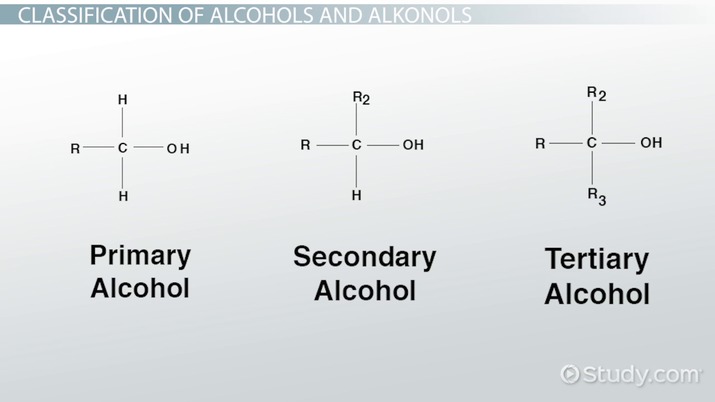
“R” is an abbreviation for a radical (unidentified ). But assume it to be a bonded carbon atom instead.
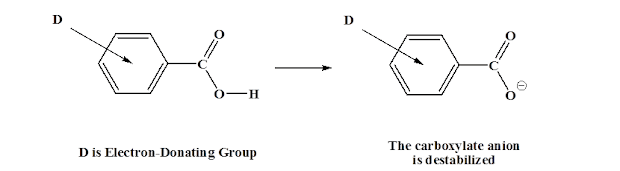
Carboxylic Acids
Organic acids such as are defined by the presence of the carboxyl functional group, . Wherein is an unidentified .

This compound has both hydrogen bonding and an extra dipole-dipole force. Because it is stable (resonance structure), the can be donated. **Note the becoming .
Nomenclature
Carboxylic acid the the most important functional group. In the alkane parent name, replace -e with -oic acid. The first carbon is always the carbon that is part of the carboxyl group.
2,2-dichloro-3-ethyl-5-hydroxyl-5-iodo-4-phenylhex-3-enoic acid
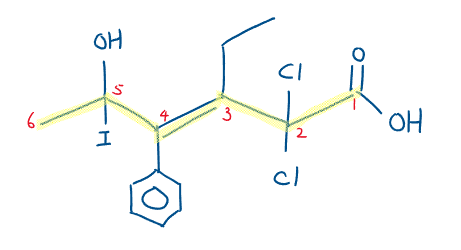
Esters
Esters are formed from an esterification reaction (a type of condensation reaction) AKA Dehydration synthesis. Esterification involves combining and alcohol and a carboxylic acid.
The of the carboxylic acid and the of the alcohol to form water. Bonding the single bonded to the carbon that was previously bonded to the hydroxyl group of the alcohol.
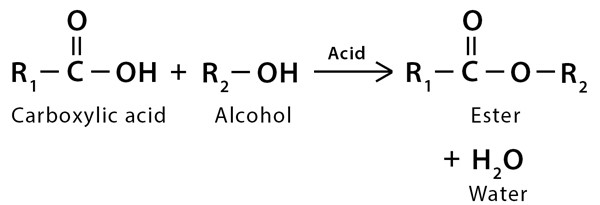
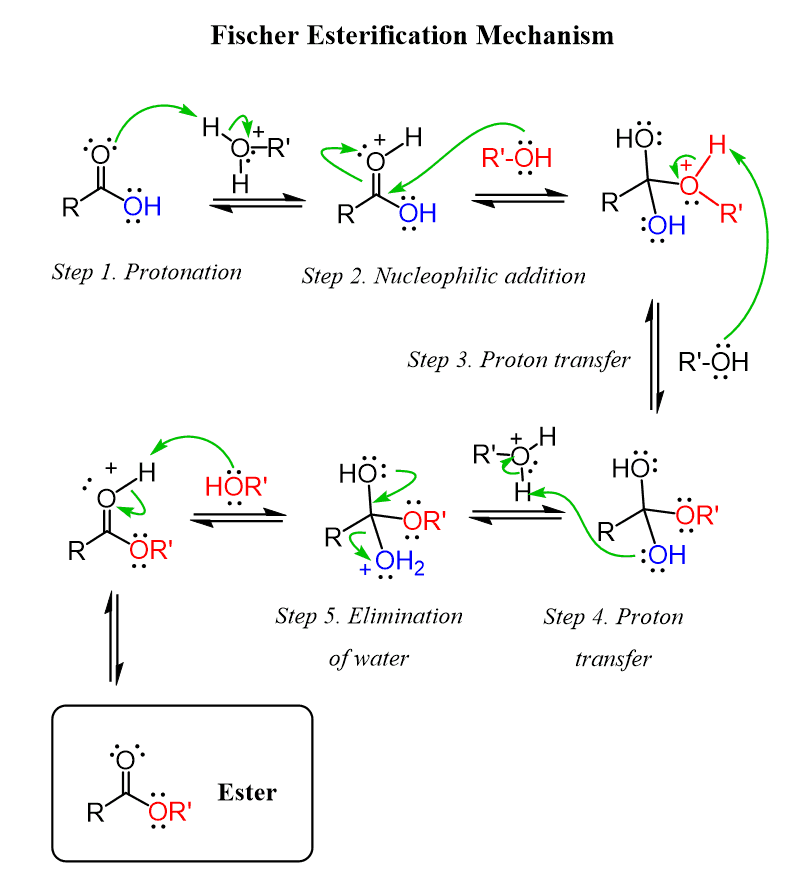
Nomenclature and Reaction
Take the alcohol name, place it first and give it the alkyl name: e.g. methanol → methyl. Add a space and then turn the acid into the parent molecule. Replace the -oic acid with -oate: e.g. ethanoic acid → ethanoate.
Methanol + ethanoic acid → Methyl ethanoate + water.
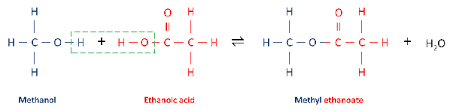
Properties
They general are responsible for odor and taste in natural and synthetics flavors and smells.
Amines
Use extensively in medicines. Alkaloids are a type of amine found in plants. Opium alkaloids are used s painkillers, highly addictive. Putrescine is a polyamine responsible for the smell of decaying fish (amines generally smell bad). An amine is a relative of ammonia - it is composed of one or more hydrocarbon chains that are attached to a nitrogen.
Generic formula: . The amine is the portion.

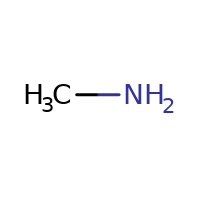
Degree 1 (Primary) amines
Degree 1 (primary) amines only have one hydrocarbon chain attached. An example of such an amine is methanamine (old name = methylamine)
- Contains hydrogen bonding because of the bonding
Degree 2 (Secondary) Amines
Degree 2 or secondary amines have two hydrocarbon groups. The longest chain containing the nitrogen becomes the parent name. An ‘N’ goes before a branch name if it is attached to the nitrogen. For example: N-methyl-2-methylbutamine.
- Contains hydrogen bonding
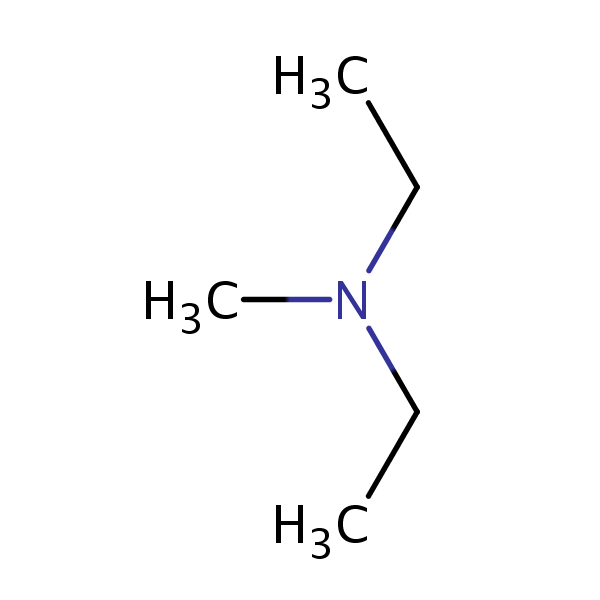
Degree 3 (Tertiary) Amines
Degree 3 or tertiary amines have three hydrocarbon groups. For example: N-ethyl-N-methylethanamine. Or: N,N-dimethylethanamine
- Cannot have hydrogen bonding with itself but because of the nitrogen’s high polarity, it will have hydrogen bonding with polar substances such as water.

Physical properties
Degree 1, and degree 2 amines contain hydrogen bonding. Degree 3 amine cannot hydrogen bond with itself, but will hydrogen bond with water. All amines do dissolve in water, with smaller amines dissolving better.
- ethanol >> pentanol
- ethnamine >> pentamine
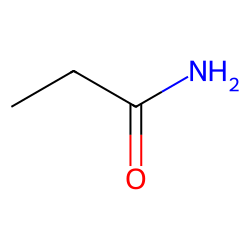
Amides
A carboxyl group ( is formed from a carboxylic acid and is attached to nitrogen. Thus it is more closely related to carboxylic acids than to amines. The most important amides are proteins.
Change the acid name, replace ic/-oic acid with -amide. For example: Propanoic acid → propanamide.
Degrees
Degrees of amides are the same as amines. N-Methyl propanamide - Degree=2 N-ethyl-N-methyl butanamide Degree = 3
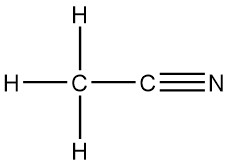
Nitriles (Cyanides)
Compounds with group are often not considered as an organic group because they can break off into a polyatomic ion. So if the cyanide is acting as an ion it is an ion, however, if it is not breaking off or bonded with a metal it is considered organic. It has a set of rules, a branch name of -nitrile or (older naming branch: cyanide). For example ethanenitrile:
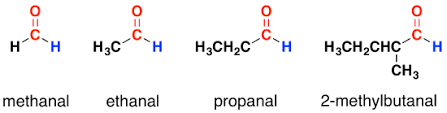
Aldehydes
Aldehydes and ketones have a carbonyl group . Where aldehydes always have their carbonyl group on the terminal carbon atom. Aldehydes are products of alcohol decomposition.
- There are no possible cyclic aldehydes
- Naming as alkane, drop the -e and replace it with -al Methanal = aka formaldehyde, Ethanal = aka acetaldehyde, Proponal
Formaldehyde is what is used as a biological preservative. Our bodies -re very susceptible to this because it is small and very polar, reacting water with formaldehyde. Essentially, this process dehydrates cells.
In recent years, this has fallen out of favor as it is toxic - replaced by resin. Small aldehydes have unpleasant and irritating odors. Odor also lingers. Larger aldehydes have nice smells and are considered critical components of perfumes. Odor also lingers. Aldehydes can continue to oxidize into carboxylic acids.
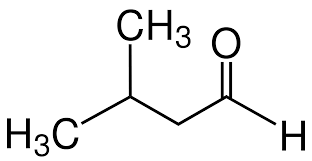
Ex.3-methylbutanal
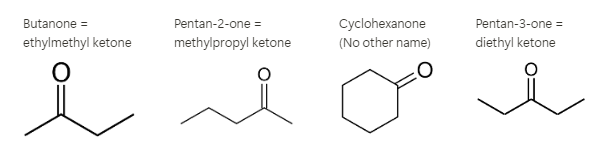
Ketones
The carbonyl group can only be found inside a chain. The carbonyl group cannot be found at a terminal. Note that you can’t have a cyclic ketone or aldehyde -terminal =midpoint. The smallest possible ketone is propanone, Naming - Name as an alkane and replace -e with -one Name as alkyd groups around ketone: Propanone can be named: Propanone = dimethyl ketone or colloquially ace Acetone

- Some characteristics of ketones: Decadent (they make your hands feel crusty).
- Good organic solvent
- Good for removing sticker residue
- Acetone tries to dissolve some plastic
- A note about ketones is that the process of ketosis converts fat to energy + ketones in a sugar deficit. This diet cuts out all carbs so that fats can be broken down for energy and as ketones as a byproduct. Ketones are known for having a foul odor and are also responsible for ‘morning breath’ (ketosis and digestion are prevalent during sleep).
Ethers
The old naming convention had one ‘side’ or branch of the ether as an alkyl, with the other side named in the same manner, and terminating the name with, ‘ether’. E.g. dimethyl (methyl methyl → dimethyl) ether or more generally, alkyl alkyl ether. The updated name for these chose the longest branch as the main branch (if tied, choosing one is arbitrary) and having the other branch as alkoxy as the branch name. E.g. dimethyl ether would be methoxy ethane.
Ethers are characterized by having a single bonded with alkyl groups.

Special Names for Special Branches
Special names are given to specific configurations of branches to make naming more precise.
- Iso propyl has a carbon attached at its ‘diverging carbon’ - meaning that it has the minimum amount of paths to diverge (given it is not just some branch).
- Isobutyl is similar to isopropyl in that it has its diverging carbon into two (the minimum paths).
- Sec-butyl (dash is necessary) has a diverging carbon first but still retains the two paths.
- Tert-butyl has a diverging carbon immediately, while also having three diverging paths.

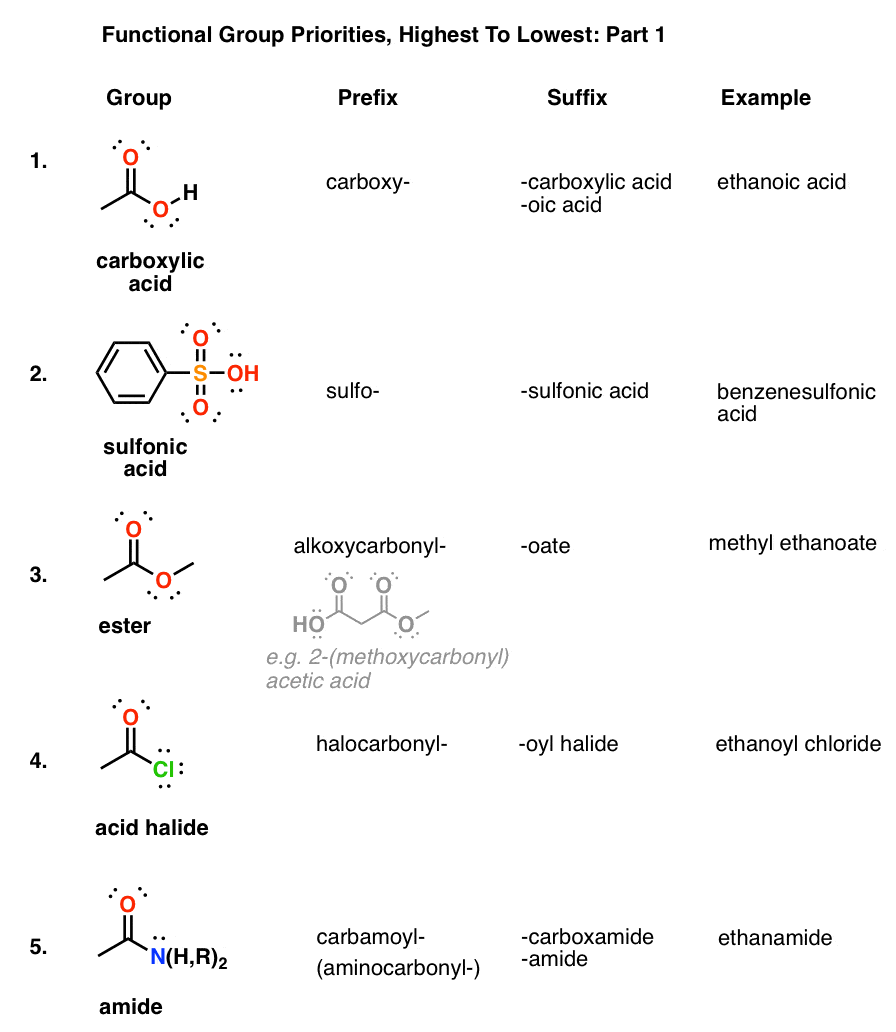
Functional group priority (Lewis definition)
The Lewis definition of functional group priority makes the ‘most acidic’ have the greatest priority:
- Carboxylic acid
- Ester (close to carboxylic acid)
- Amides (close to carboxylic acid)
- Nitriles
- Aldehydes
- Ketones
- Alcohols
- Amines
- Ethers
- ,,, Alkene, alkyne,,,
- Alkyl halides
- Alkyl branches/alkane
Mnemonic: Oxy>Est>Ide = Oxyestide Nit>Hyde>Ones = Nick hides one’s Alc >Mine = Alcohol (mine) Eth>Ene>Yne = Ethene/ethyne Hal>Branch = Hall’s branch = Oxyestide, nick hydes alcohol mine ethene/yne in the hall’s branch.
Physical Properties & Summary
Boiling Point & Melting point (Alberta curriculum)
Intermolecular Forces (IMF)
LDF = London Dispersion Force: DD = Dipole-Dipole force: HB = Hydrogen bond:
Shape & Branching
The more linear/less branches an organic molecule has, the stronger the IMF. The more spherical/more branches an organic molecule has, the weaker the IMF. *Alcohols and carboxylic acids tend to have the highest boiling points
| LDF | DD | HB | |
|---|---|---|---|
| Alkanes (Aliphatic) | ✓ | ||
| Alkenes (Aliphatic) | ✓ | ||
| Alkynes (Aliphatic) | ✓ | ||
| Arene, aromantic, benzene (Aliphatic) | ✓ | ||
| Alkyl halides | ✓ | ✓ | |
| Alcohol | ✓ | ✓ | ✓✓✓ |
| Carboxylic acids | ✓ | ✓✓ | ✓✓✓ |
| Esters | ✓ | ✓✓ | |
  |