2024-07-1616:54 Status:IBnotes
Structural/Constitutional Isomers
Structural or constitutional isomers have completely different structures and names. Any isomer that exists but shares the same quantity of atoms (having the same molecular formula) but differing structures are structural isomers. For example: hexane, 2-methylpentane, 3-methylpentane, 2-3,dimethylbutane, and 2,2-dimethylbutane are all structural isomers of each other. They all share the same molecular formula of:
Stereoisomers
Stereoisomers are isomers that only differ by how atoms are arranged in 3-dimentional space. They differ from structural/constitutional isomers as they can have the same 2D structures, often sharing names, but are different spatially. There are two kinds of stereoisomers: configurational and conformational isomers (with additional subcategories).
Conformational Isomers
Conformational isomers are isomers that are ‘rotated.’ Newman diagrams are often used to depict how conformational isomers can be differentiated from each other. The lines ‘behind’ the circle represent the bonds to the carbon behind the first; the lines ‘in front’ of the circle represent the bonds attached to the first carbon. The angle of these lines determines the kind of conformation the isomer has. Another way to do this is by using a seesaw diagram (this is less common) and more intuitive.
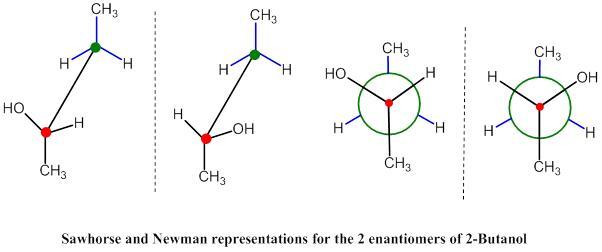
The diagram on the left is a seesaw diagram, whereas the one on the right is a Newman diagram. Newman diagrams will be used to express fully eclipsed, gauche, eclipsed and anti-staggered conformations.
There are theoretically infinite amounts of conformational isomers, however, there are certain conformations that are more likely: Fully eclipsed configurations are unlikely because the bonded orbitals occupy a lot of space, and having the strongest/largest orbitals ‘overlapping’ is unlikely as the electrons would repel each other. An eclipsed conformation is any one that ‘overlaps.’ A staggered conformation is one that leaves space for orbitals. A gauche position is a staggered position that keeps the bonds apart from each other but does not have the most electronegative/strongest bonds apart. An anti-staggered conformation has the greatest distance of the strongest bonds. These tend to be the most stable conformations.
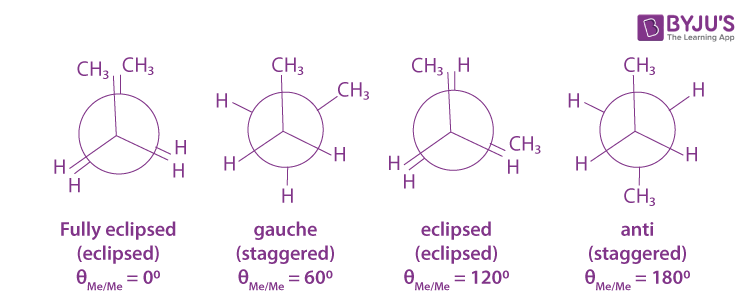
Conformational isomers can be made by rotating a single bond. At standard conditions, it can be assumed that bonds are constantly rotating
Configurational Isomers
Geometric
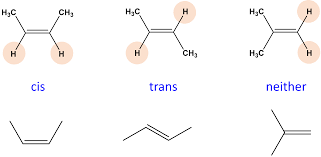
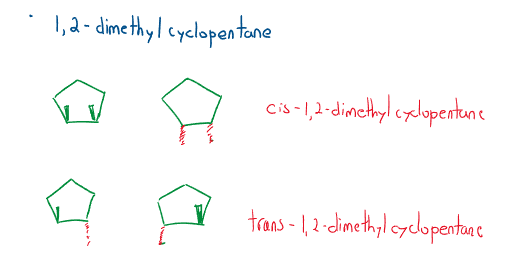
Cis/trans definition
Geometric or configurational isomers are isomers that have the same overall structure but have some of the atoms arranged in different locations.
- cis-: has “groups” on the same side of a bond.
- trans- : has “groups” on the opposite side of a bond.
Where “groups” are generally alkyl branches or halogen branches. *Wedges must be used for cyclic geometric isomers
.
Geometric isomers have different physical and chemical properties, because they have fixed bonds that cannot rotate and therefore the isomer has to act in a specific way (isomer transformation is not possible).
There is a limitation as the cis/trans denotation only works when there are two groups.
(E)/(Z) Definition
Because of the limitation of the cis/trans definition, priority is assigned to all the atoms attached. Priority is based on atomic number (the greater the number, the higher the priority). If the atomic numbers are tied, the atom attached determines the principle atom’s priority.
If the highest priority atoms are on the same side, the molecule gets a (Z) denotation. If the highest priority atoms are on the opposite sides, the molecule gets an (E) denotation.
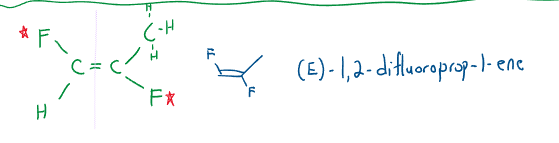
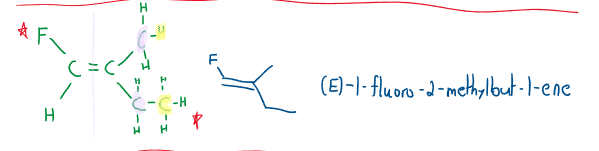
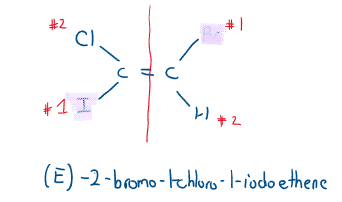
Watch out for the following when naming
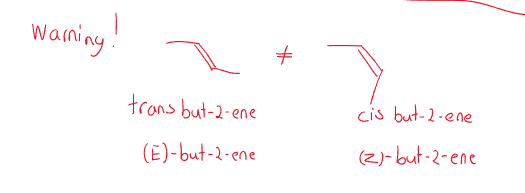
Optical Isomers
To determine whether or not an optical isomer is an enantiomer or a diastereomers, check the chirality of the molecule. Chirality can be determined by looking at the reflection. If the reflection can be superimposed (can be placed on top of each other or eclipsed) then the molecule is achiral. If the mirror image cannot be superimposed, then it is chiral. Some examples of chiral vs. achiral objects:
- Spade = achiral
- Car = chiral
- Cat = chiral
- Corkscrew = chiral
- Screwdriver = achiral
- Bicycle = chiral
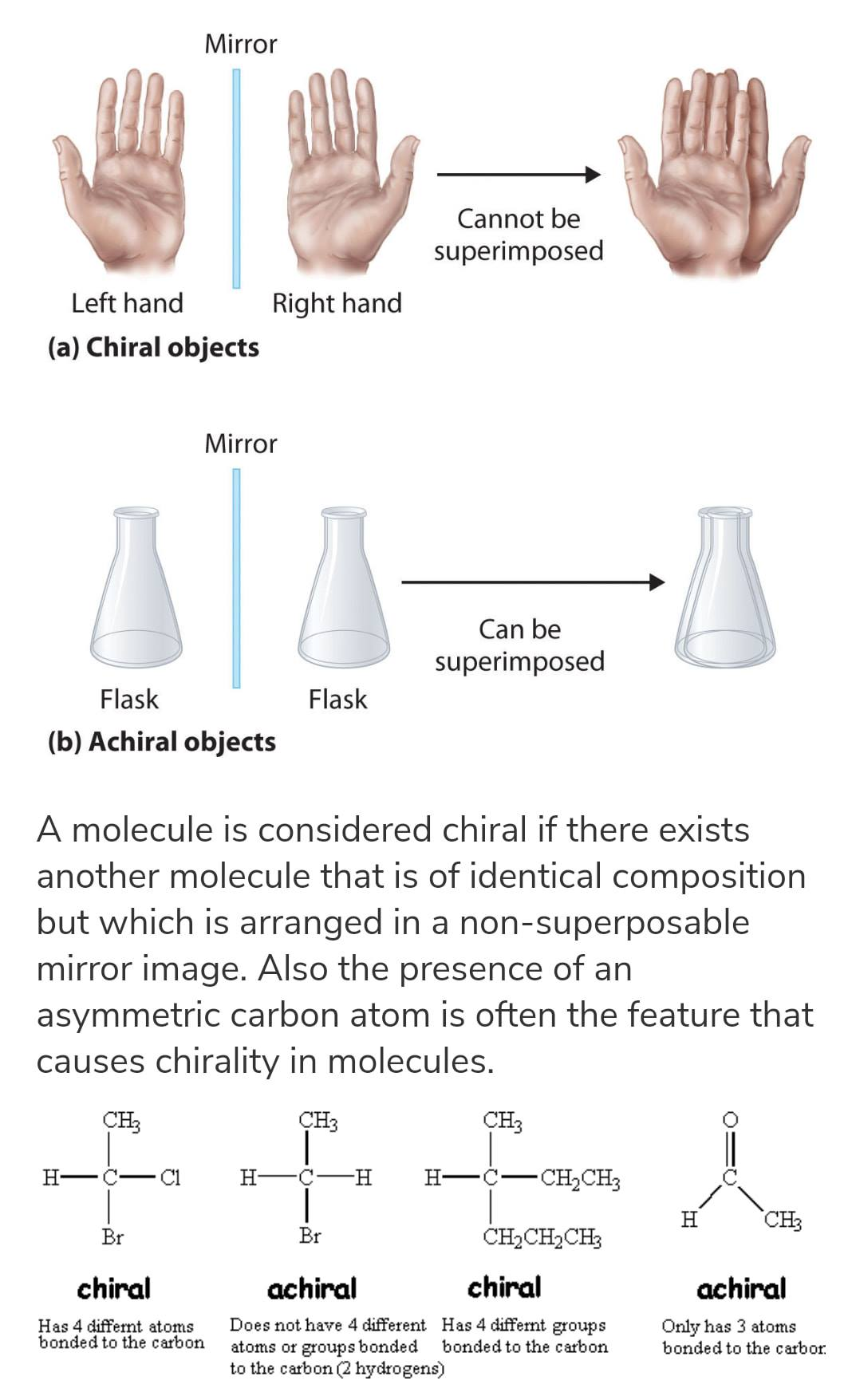
Chiral Centers (Carbons) A chiral center is a carbon that has four distinct groups bonded, and is nonsuperimposable. If it is in a cyclic structure, then draw a line of symmetry and see if the groups on wither side of the carbon are the same.
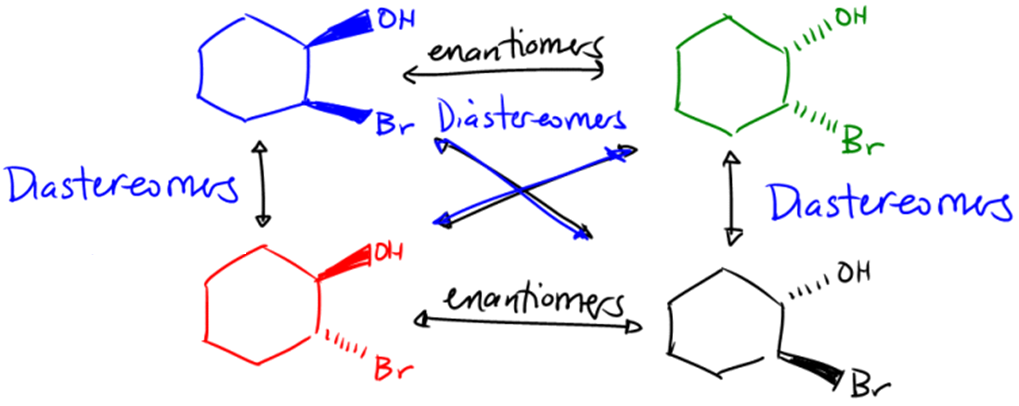
Enantiomers Enantiomers are a pair of molecules that are nonsuperimposable images of each other. Most of these molecules have a chiral center.
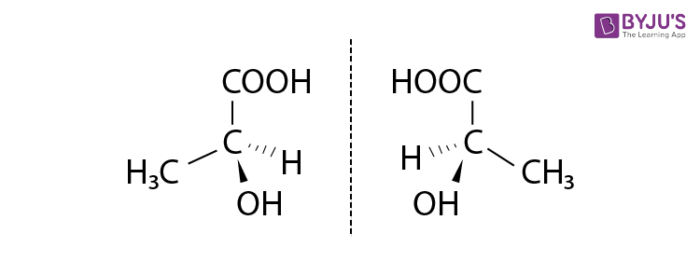
- Physical and chemical properties of enantiomers are very similar. They are essentially identical in chemical reactions.
- However biologically, most enzymes are chiral in nature and so when chiral molecules interact with other chiral molecules there is a significant difference: For nutrience sugars are a good example, for pharmaceutical applications, thalamide is a good example.
Diastereomers
Diastereomers are a pair of molecules that are non-mirror images of each other and often have multiple chiral centers. These have to be restricted in rotation.
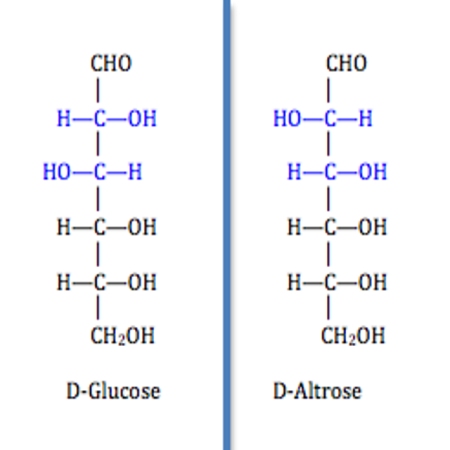
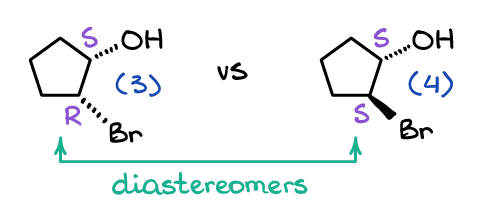
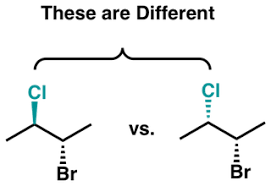
Optical Activity Chiral molecules are optically active, they can rotate a plan of polarized light.
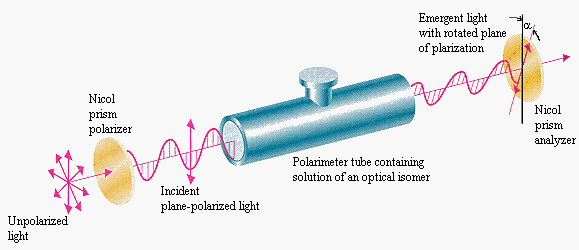
A racemic mixture is composed of a 50:50 mixture of two enantiomers, and have no optical activity:
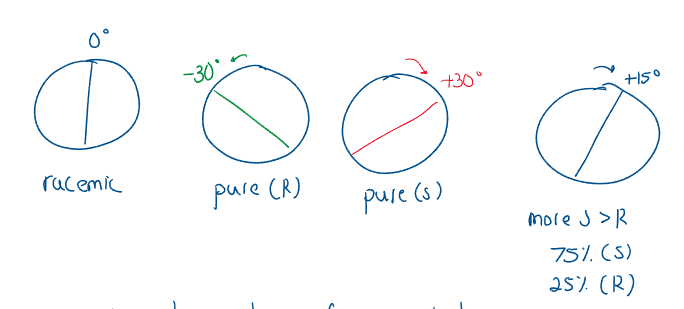
When the optical activity of pure substances are known, the relative percentage of the mixtures can be determined