2024-07-1015:13 Status:IBnotes Tags: Thermodynamics
Energy
Energy is the ability to do work. Work being the process of moving matter. Energy, like mass, cannot be created nor destroyed. That is to say, that the amount of energy in the universe is finite.
Kinetic energy
Energy associated with motion is kinetic energy. Thermal energy and temperature fall under this category, as the temperature of a substance is the ‘average’ speed at which a molecule is moving/vibrating.
Chemical potential energy
Chemical potential energy is the energy stored in the chemical bonds of a substance. The breaking and forming of bonds both release or store chemical potential within/through their bonds. Furthermore, definitions of endothermic and exothermic reaction have to do with a change in chemical potential. A significant part of thermochemistry is directly due to chemical potential.
Calorimetry
Bomb calorimetry
This is a unique kind of calorimetry that is utilized because the use of water as the calorimetry does not work. For instance explosions and combustions would not be accurately measured because there is another form of energy involved . E.g. Light energy being emitted from a Mg.
A bomb calorimeter encloses the reaction inside a sealed container → placed inside a calorimeter Bomb calorimeter combines mass and specific heat capacity into one value denoted as capital C If the question specifically specifies a bomb calorimeter use C instead of c and m. (C = J/degree C) Assume that all energy goes directly into/out of the system as thermal energy (for problems involving temperature).
When the change in enthalpy is positive, the reaction is endothermic (as the system gains energy from the surroundings.) When the change in enthalpy for the reaction is negative, the system is losing energy and the surroundings are gaining energy. Ensure that this is reflected before using .
Enthalpies of Solutions + Tips
When neutralization reactions occur (between strong acids and strong bases) an exothermic reaction occurs. As aqueous solution are mostly water it is reasonable to assume that the specific heat capacity of the solution is equivalent to that if water, . Additionally the weight of the solute does not affect the overall mass of solution.** Always write equation and all steps for chem questions.
Communicating Change in Enthalpy
- The can be written inside the equation:
- The can be written outside the equation:
- The of each substance can be written:
- The relative positions of reactants and products as it relates to their enthalpy can be shown (graphs)
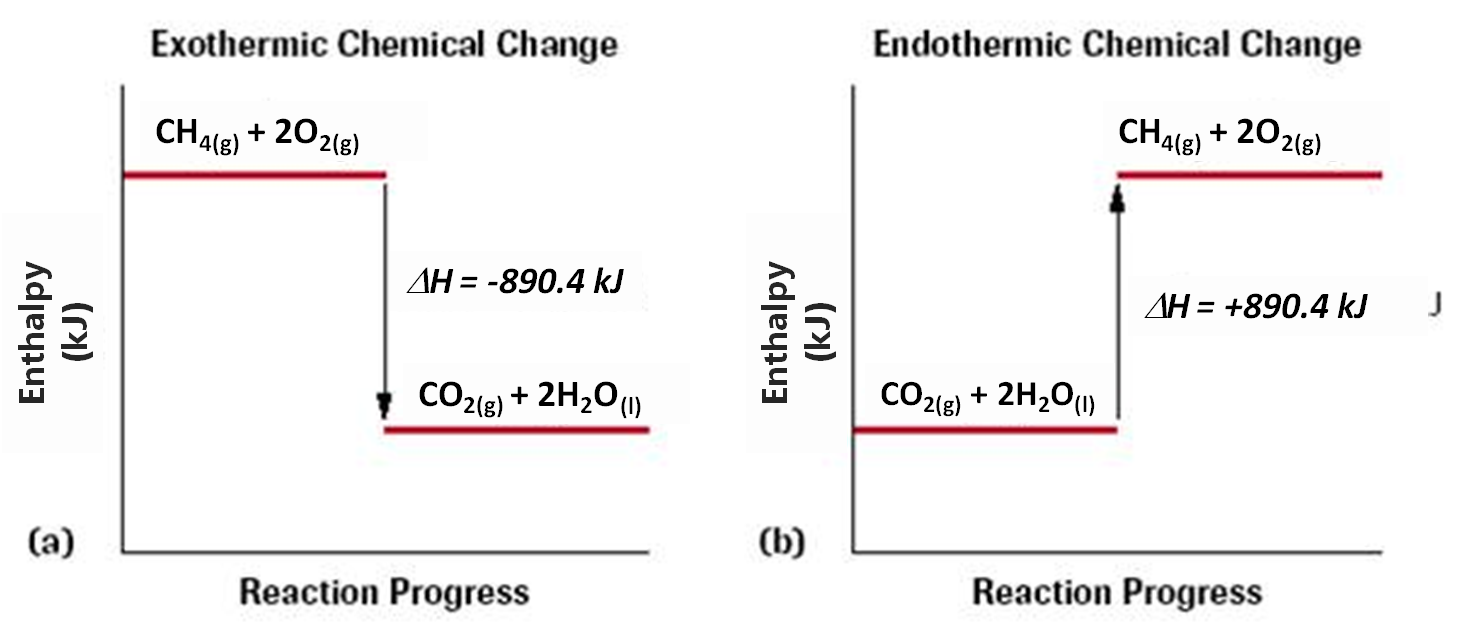 This does not account for activation energy
This does not account for activation energy
Standard Molar Enthalpy
In order for chemists to the same page regarding molar enthalpies, standard molar enthalpy are used such that results of experiments are consistent. To denote a standard change in enthalpy , and are used to show that the enthalpies are standard. Measurements are taken in the following conditions:
- Pressure =
- Concentration of aqueous solutions =
- Ambient temperature (of surroundings) =
- Substances are in their most stable states
Hess’ Law
Change in enthalpy formula
The net enthalpy change of a reaction is the sum of the enthalpies for each reaction that is added. Change in enthalpy Delta H = H(products) - H(reactants)
This equation is communicating that the change in enthalpy (chemical potential energy) of a reaction is equal to the amount that the products have (often in a more stable state and therefore have a lower enthalpy) subtracted by the amount of ‘energy required to form the reactants.’ This results in the difference of enthalpy. The difference of enthalpy is equivalent to the energy released (if negative, energy was released; if positive, energy was absorbed from the surroundings)
The amount of moles used in a reaction can be expressed as follows
Where is the standard molar enthalpy of a substance. That is to say that when one mole of X is used/produced there is a finite change in enthalpy. It is measured in . The total change in enthalpy is where is any reaction (fusion (fw), combustion (comb), m (/mol), solution (sol), formation(f), vaporization(vap))
Puzzle Hess Example:
In order to find the enthalpies of reactions with an unknown reaction, puzzle Hess may be used. Multiplying and adding several given reactions, a target reaction can be reached.
Given equations: \begin{gather} \bullet \ \ \ 2H_{2(g)} + O_{2(g)} \rightarrow H_2O_{(g)} \ \ \ \Delta H =-538.6kJ \newline \bullet \ \ \ C_3H_{8(g)} \rightarrow 3C_{(S)} +4H_{2(g)} \ \ \ \Delta H =103.8kJ \\ \bullet \ \ \ CO_{2(g) } + 3O_{2(g)} \rightarrow 3CO_{2(g)} \ \ \ \Delta H =-393.5kJ \end{gather}
\begin{gather}(2 \times (2H_{2(g)} + O_{2(g)} \rightarrow H_2O_{(g)} \ \ \ \Delta H =-538.6kJ)) \newline (-1 \times (C_3H_{8(g)} \rightarrow 3C_{(S)} +4H_{2(g)} \ \ \ \Delta H =103.8kJ)) \\ + \ (-3 \times (CO_{2(g) } + 3O_{2(g)} \rightarrow 3CO_{2(g)} \ \ \ \Delta H =-393.5kJ) ) \\ \end{gather}
- Components of the reactions and their enthalpies are given
- Very useful for solving enthalpy reactions that cannot be measured through calorimetry for instance, the reduction of Mg, in where energy is released as light.
Formation Hess Example:
Process:
- Find the balanced equation
- Use the formation Hess formula to find the change in enthalpy per mole of one of the substances
- Account for the moles of the substance in the formula (if there are 2 moles of substance divide by 2)
- Find the amount of moles of substance and multiply it by the change in enthalpy per mole of that substance
- Use the equation to find the temperature
If of heats up 5000kg$$H_2O_{(l)}, how much does the temperature increase by? \begin{gather} 2C_8H_{18(l)} + 17O_{2(g)} \rightarrow18H_2O_{(g)} +16CO_{2(g)} \\ \ \\ \Delta H \degree_{rxn} = \Sigma n\Delta H\degree_{products} - \Sigma n\Delta H\degree_{reactants} \\ \Delta H \degree _{rxn} = (18(-241.8kJ+16(-393.5kJ))-(17(0)+2(-250.1kJ)) \\ \Delta H = -10148.2kJ \\ \ \\ n\Delta H\degree_{mol C_8H_{18(l)}} = \Delta H_{comb} \\ \Delta H\degree_{mol C_8H_{18(l)}} = \frac {-10148.2 \frac {kJ}{mol}}{2 \ mol\ C_8H_{18(l)} } \\ \Delta H\degree_{mol C_8H_{18(l)}}=-5074.1kJ \\ \ \\ n H\degree_{mol C_8H_{18(l)}}=-mc\Delta T \\ \Delta T = \frac {\Delta H \degree _{mol_{C_8H_{18(l)}}} \times n}{mc}\\ \Delta T = \frac {(15 kg \times \frac {1000g} {1kg} \times \frac {1 \ mol_{C_8H_{18(l)}}}{114.26g} )\times (-5074.1kJ \times \frac {1000J} {1kJ} )} {(5000kg \times \frac {1000 \ g} {1kg} ) (4.19 \frac J {g\degree C}} \\ \Delta T = + 31.79596674 \degree C = +32\degree C \end{gather}
- Formation enthalpy of the reactant and products can be combined to get net enthalpy (IB doesn’t like this method - they prefer bond enthalpies)
Signage:
- If we reverse the direction of the reaction, amount of enthalpy change is constant, but the change in enthalpy is opposite in sign, but the same in magnitude.
- If we multiply a complete reaction by a coefficient, then the reaction must also be multiplied by the coefficient.
Other things of note:
For open combustion (ventilated fires) - assume water vapour is formed For closed combustions - assume water liquid is formed (bomb calorimetry, cellular respiration) Elements do not have a formation enthalpy
Activation Energy
All reactions require energy to commence- this is the activation energy. (Fires don’t start themselves). Even if the materials are highly reactive, they still require the activation energy to be reached. They require a ‘push’ such as ignition or mixing. Depending on the reaction, the ‘push’ can be very small or large (relatively.)
Energy is needed to break bonds (they MUST be broken first.) Once there is an incident bond broken, the energy released, typically, provides enough energy for the reaction to be self-sustaining. The energy required to ‘start’ the reaction, the activation energy is denoted by . This is an all or nothing value. That is to say that unless this threshold is reached, the reaction will not start.
A more accurate depiction of the graph depicted for change in enthalpy includes the activation energy.
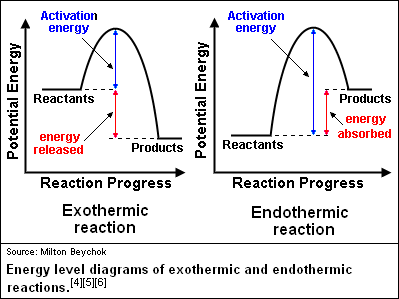
Catalysts:
A catalyst is a substance that increases the rate of a chemical reaction. It is also not consumed in the reaction and can be used repeatedly. A catalyst provides an alternate pathway for the chemical reaction to occur, resulting in a lower activation energy. (Because catalysts ‘help the reaction happen,’ they lower the ‘load’ or energy required for the reaction to take place.) Examples of catalysts include:
- Catalytic converters in cars (the catalyst, and is found in the exhaust, makes fossil fuels less harmful, turns into , and turns into .)
- Oil and case industry use catalysts to refine crude oil.
- Catalysts are often used in industry to speed up reactions and can also help increase the yield of a product.
- Enzymes are an example of biological catalyst.
- They exist in living systems and help speed up biochemical processes
- Enzymes are proteins
- Enzymes and other catalysts in the body work best at = body temperature
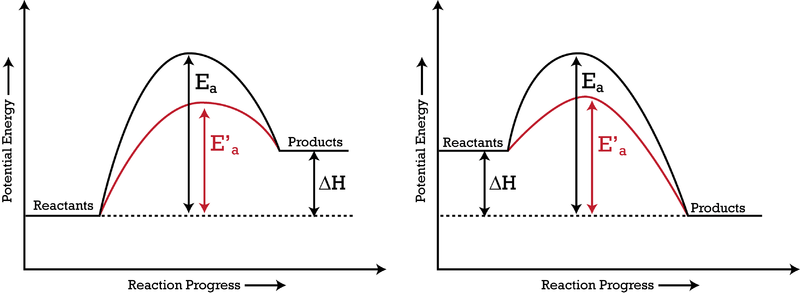
In the right graph above, the activation energies are incorrect - (subtract their current values by )
IB note: Catalysts are temperature sensitive (dependent on context.) e.g. starting a fire while the surroundings are hot is easier than trying to start a fire while the surroundings are cold.
Multistep thermochemistry (recap of formulas)
Theory here is redundant, do problems.
Bond Enthalpies
Bond formation releases energy, bond breaking absorbs energy. Information here can be found in Table 2 of the IB data booklet. For these kinds of questions, it is very helpful to draw diagrams of all the molecules formed and broken.
Example 1 of applying bond enthalpies:
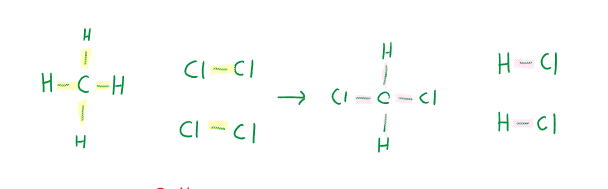 \begin{gather} \Delta H_r = [4mol (414kJ/mol)_{C-H } + 2mol (431 kJ/mol)_{H-Cl}] \\ - [2mol(324 kJ/mol) _{C-Cl } + 2mol (414kJ/mol)_{C-H} + 2mol (431kJ/mol)_{H-Cl)]} \\ = -198kJ \end{gather}
\begin{gather} \Delta H_r = [4mol (414kJ/mol)_{C-H } + 2mol (431 kJ/mol)_{H-Cl}] \\ - [2mol(324 kJ/mol) _{C-Cl } + 2mol (414kJ/mol)_{C-H} + 2mol (431kJ/mol)_{H-Cl)]} \\ = -198kJ \end{gather}
Example 2 of applying bond enthalpies:
“Average” bond enthalpy
The bond enthalpies have slight differences depending on the conditions in which it is found. For instance, bond found in and would all have different bond enthalpies depending on the the other atoms bonded. Respectfully, having a bond enthalpy of and , the average bond enthalpy for a bond is , (assuming that all variants are equal in relative quantity.)
Ozone
The ozone layer is a chemically active layer of gas in our atmosphere/stratosphere - composed of and . Both play a role in protecting the earth from harmful radiation from the sun (Look at what intense UV and IR radiation can do to a planet like Mars for an example)
Recall the oxygen gas has a full double bond (bond order of two.) Ozone shows a resonance structure where the double bond exists in multiple location - There is a semi double bond (This produces a bond order of 1.5) - Ignore hydroxide.
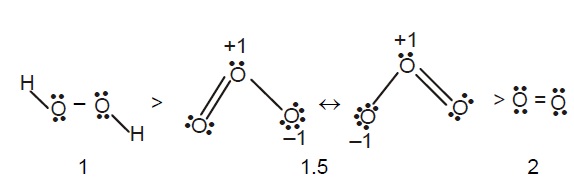
The bonds in both molecules can be broken by certain photons with sufficient energy. (If the fundamental frequency is broken)
Example of breaking bonds with sufficient energy:
The bond enthalpy in oxygen is 498kJ/mol and for ozone it is 363kJ/mol. Calculate the wavelength of light capable of breaking these bonds, and identify the type of light. (The formulas below can be found in Table 1, 12.1, and 2.2)
Where is the wavelength, is Planck’s constant, ,
\begin{gather} 498\frac{kJ} {mol} \times 6.022 \times \frac {mol} {6.022 \times 10^{23} } = 8.27\times 10 ^{-22} kJ = 8.27 \times 10 ^{-19} J \\ \lambda = \frac {hc} E = \frac {(6.63 \times 10^{-34}) (3.00 \times 10^{8}m/s)}{8.27 \times 10 ^{-19}J}\\ \lambda_{O_2} =2.41 \times 10^-9 \\\lambda_{O_3} = 3.30 \times 10^{-9} \\ = UV \ radiation \end{gather}
Free Radical Cascade of UV (Chain reaction)
Ozone Oxygen Cycle
When UV radiation breaks the bond of an abundant molecule, it produces two free radical oxygen atoms, . This is an endothermic reaction as light is absorbed and breaks the bonds. When is produced, it is highly reactive. And has a tendency to react with the abundant, molecules present. This produces and is also an endothermic process. Finally, energy is released, when is broken into and . This is a complete cycle. In addition, can react with these oxygen radicals or the oxygen radicals can terminate. There is a small net loss in the layer, but it is replenished by air circulation, and the cycle persists.
Chlorofluorocarbons
Free radial contaminants, such as chlorofluorocarbons, act as catalysts and leads to rapid ozone depletion. have a very light density and are susceptible to UV breakage. This cycle is likely to continue because ozone is the most prevalent in the ozone layer. ( is the least likely to form because there is not much) Thus, is depleted at a rapid rate, leading to an increased intensity of UV and IR radiation.
Energy cycles
The full cycle, the Born Haber cycle can be illustrated as in the diagram below or by utilizing the following formula.
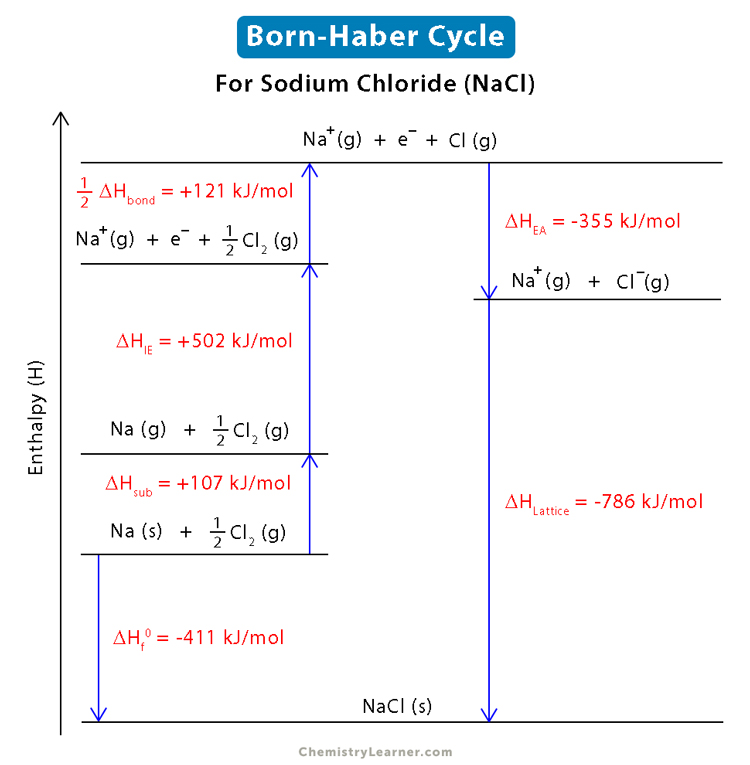
The enthalpy of formation, is equal to the change in enthalpy when all substances are turned into gas via sublimation or broken . Sublimation enthalpy will be given whereas, bond enthalpies will be in table 11. Once, the molecules have been sublimed, they must have an electron taken from them or taken away. The energy required to remove an electron, and the energy gained from adding an electron, need to be accounted for as well. Note that for every electron gained or removed, has a different ionization energy and electron affinity. (First, second etc.) Finally, the lattice enthalpy, is typically the amount of energy gained when a lattice is formed. However, IB makes enthalpy positive, defining it as the energy required to break the lattice (endo).)
The enthalpy of solution/hydration
- The enthalpy of solution can be measured through calorimetry.
- One mole of solution at SATP conditions are used to quantify this value.
- These reactions are endothermic and exothermic. They are contingent on the interactions between ions and water. Hydration shells create very stable structures with ions.
Entropy
Entropy is a measurement of order/disorder, a measurement of ‘randomness’ or chaos, or pure information. And can be denoted by
Second Law of Thermodynamics
The formula above only applies to the net of the universe as a whole. And the entropy of the universe is always increasing. Whenever a system has become more disordered the entropy increases and is positive. ‘Chaos is the inability to discern a pattern.’
- It is possible to decrease entropy (increase order) of a system but in turn the must increase in greater magnitude.
- Entropy is proportional to enthalpy, heating up temperature, increases entropy.
Third Law of Thermodynamics
The entropy of a pure crystalline substance at absolute zero, () is also. (Absolute zero is purely theoretical - atoms are defined by their vibrations)
- The randomness/entropy decreases as temperature decreases
- Even in a solid, on the same atomic level, there is movement and oscillation.
- This cannot be observed because of a scale issue.
Entropy and Spontaneity
Knowing enthalpy and entropy can help us determine whether or not a reaction will be spontaneous. Spontaneity is a description of the possibility to occur, without continued external interference (energy or work.) - Self sustaining = spontaneous. A non-spontaneous reaction, would require a constant work done externally to occur. (Constantly needs to be ignited)
Spontaneity had nothing to do with reaction rate/activation energy/speed/sensitivity/volatility of reaction.
A process that is spontaneous in one direction is not spontaneous in the other direction.
- Cell respiration is spontaneous, whereas photosynthesis requires constant work (not spontaneous)
- You can cook an egg to where it is spontaneous and self sustaining, then it is theoretically possible to ‘uncook’ that egg with enough constant energy/work.
Quantitative changes in enthalpy
= Entropy of one mole of a substance at standard conditions. The units of which are
Example: Calculate the for the following
System to System Comparison (Qualitative Changes in Enthalpy I)
Temperature change - since faster molecules are more random.
Phase change - going from solid to gas increase in entropy. ()
Dissolving - solid → dissolve in water → aq (), gas → dissolve in water → aq ()
Increasing the number of (compare the number of moles of product vs reactants (if 1 mol of produces , then the also increase. As there are more ‘things’ that are moving around)
Molecule to Molecule Comparison (Qualitative Changes in Enthalpy II)
Entropy change generally increases with mass (’increased complexity) of the particles.
When predicting reactions, all the system system reactions are more important than the individual complexities of each molecule.
Gibbs Free Energy
= change in Gibbs free energy, the maximum work that a system can do OR the minimum amount of work must be done. = change in enthalpy, = change in entropy, is the temperature, .
- = spontaneous reaction
- = process at equilibrium
- = non-spontaneous reaction
Free energy is a state function - only initial and final conditions matter.
- If a reaction has then it is an enthalpy driven reaction. That is to say that if the magnitude of the enthalpy is greater, than it has more influence in the reaction. Meaning that the reaction likely happed as a result of enthalpy rather than entropy
- If a reaction has then it is an entropy driven reaction. That is to say that the magnitude of the entropy is greater than the enthalpy. Meaning that the reaction more likely happened as a result of entropy rather than enthalpy.
Types of reactions:
- (exothermic), (messy) → Spontaneous at all temperatures
- (endothermic), (tidy) → Non-spontaneous at all temperatures
- (exothermic), (tidy) → Spontaneous at “low” temperatures
- (endothermic), (messy) → Spontaneous at high temperatures
Standard Free Energy of Formation (
Note that the standard free energy for formation of an element is 0.
Example of a Standard Free Gibb question:
Since , this reaction is spontaneous.