2024-07-1113:07 Status:IBnotes
Introduction to Electrochemistry & redox
An electrochemical reaction occurs when there is a transfer of electrons between materials. One substance gains electrons and another loses them.
Oxidation and Reduction Agents (RA)
Oxidation occurs when there is a loss of electrons. This can be memorized using the OIL RIG acronym: Loss of electrons =Oxidation (OIL). If a species is oxidizing, it is forcing the ‘other’ species to reduce such that it can lose electrons. Thus the species is acting as a reducing agent, RA. This can be memorized using the following: Reducing Agents Gain electrons, (RAG).
Reducing agents are often solid metals.
Example of a Reduction Half Reaction
The following is considered to be one of the strongest reduction half reactions/strongest OAs: This makes sense, as is the most electronegative substance it would have a strong tendency towards gaining electrons. This implies that the less electronegative a substance is, the weaker its reduction half reaction, and its strength as an OA:
Reduction and Oxidizing Agents (OA)
Reduction occurs when there is a loss of electrons. This can be memorized using the OIL RIG acronym: Gain of electrons = Reduction (RIG).
If a species is reducing, it is forcing the ‘other’ species to oxidize such that it can gain electrons. Thus the species is acting as an oxidizing agent, OA. This can be memorized using the following: Oxidizing Agents Lose electrons, (OAL). Oxidizing agents are often metal ions.
Oxidation Half Reactions
If the strength of an OA is inversely proportional to its strength as a RA, then the less electronegative a species is, the stronger it will be as a RA. (Weak RA = Strong OA, Weak OA = Strong RA). Therefore the following is considered to be a strong oxidizing half reaction/RA:
As is a strong OA, it is a weak RA as these species do not want to lose their electrons as they are in a more stable state than otherwise:
Redox
For an electrochemical reaction, reduction-oxidation, redox,, reaction to occur, two opposite half reactions must take place. (Some species has to be reduced and another must oxidize) The number of electrons gained must be equal to the electron lost of two species.
Spontaneity Rule
In order for a redox reaction to be spontaneous the oxidizing agent and reducing agent have to ‘want’ to exchange electrons.
- Spontaneous: The OA must be higher in position (in the data book) than the RA
- Non spontaneous: The RA is higher or at the same position as the OA
Example 1
What is the redox reaction occurring between solid zinc and gold(III) nitrate solution? Is the reactions spontaneous? - Write the net ionic equation and compare it to the two half reactions that are taking place.
\begin{gather} 3Zn_{(s)} + 2Au(NO_3)_{3(aq)} \to 3Zn(NO_3)_{2(aq)} + 2Au_{(s)} \\ 3Zn_{(s)} \to 3Zn_{(aq)} ^{2+} \ \ \ \| \ \ \ Zn _{(s)} \to Zn_{(aq)} ^{2+} + 2e^- \ \ \ \therefore Zn_{(s)} = RA \\2Au_{(aq)} ^{3+} \to 2Au_{(s)} \ \ \| \ \ Au^{3+}_{(aq)} + 3e^- \to Au_{(s)} \ \ \ \therefore Au_{(aq)} ^{3+} = OA \end{gather}
As is ‘above’ on the table, this reaction is spontaneous.
Example 2
Determine the best oxidizing agent and reducing agent using the following table of the following reactions:
| Hg(aq)2+ | Cu(aq)2+ | Ag(aq)+ | Au(aq)3+ | |
|---|---|---|---|---|
| Hg(l) | 0 | 0 | 0 | 1 |
| Cu(s) | 1 | 0 | 1 | 1 |
| Ag(s) | 1 | 0 | 0 | 1 |
| Au(s) | 0 | 0 | 0 | 0 |
| is the best OA: It is spontaneous with all the metals so it must be ‘below’ all the other metal ions. | ||||
| is the best RA: It is spontaneous with all the metals so it must be above all the other metals. |
Example 3
Create your own reduction half reaction table using the following observations. \begin{gather} 2A^{3+} _{(aq)} +3D_{(s)} \to 3D^{2+}_{(aq)} +2A_{(s)} \\ G ^+ _{(aq)} + D_{(s)} \to no\ rxn \\ 3D^{2+}_{(aq)}+2E_{(s)} \to 3D_{(s)} + 2E^{3+} _{(aq)} \\ G+_{(aq)} + E_{(s)} \to no \ rxn \end{gather}
| OA | RA | ||
|---|---|---|---|
| 1 | (A(aq) 3+) + 3e- | ⇌ | A(s) |
| 2 | (D(aq) 2+) + 2e- | ⇌ | D(s) |
| 3 | (E(aq) 3+) + 3e- | ⇌ | E(s) |
| 4 | (G(aq) +) + e- | ⇌ | G(s) |
Writing Redox Reactions using the 5-Step Method
- List all materials/entities present:
- Include solids, liquids, gases, aqueous ions
- Include dissociated materials
- If the conditions are acidic,
- Include
- Write the actual acid and its conjugate base
- E.g:
- Write all conjugate bases for polyprotic acids
- Always include water
- Identify all materials as oxidizing agents, reducing agents or neither
- Some oxidizing and reducing agents have combinations (water and acids are common)
- Some oxidizing and reducing agents have combinations (water and acids are common)
- Identify the strongest oxidizing agent and reducing agent
- SOA: The highest in the left hand column - in the data book
- SRA: The lowest in the right hand column - in the data book
- Write the half reactions as you see in the data booklet, balance based on count.
- Write down the net ionic equation, determine spontaneity
Example 4
Can copper pipes be used to transport
| 1 | Cu(s) | H+(aq) | Cl-(aq) | H2O(l) | Cl(aq)- + H2O(l) |
|---|---|---|---|---|---|
| 2 | RA | OA | RA | OA/RA | RA |
| 3 | SRA | SOA | - | - | - |
This reaction is not spontaneous, therefore can be used to transport
Example 5
Could an aluminum can be used to store sulfuric acid?
| 1 | Al(s) | HSO4(aq)- | SO4(aq)2- | H+ | H2O(l) | (H2O(l)) + (SO4(aq) 2-) | (H(aq)+) + (SO4(aq) 2-) |
|---|---|---|---|---|---|---|---|
| 2 | RA | N | - | OA | OA/RA | OA | OA |
| 3 | SRA | - | - | - | - | - | SOA |
This reaction is spontaneous, therefore an aluminum container is a bad container.
Disproportionation
This is an equilibrium redox reaction where some species act as both the OA and the RA: \begin{gather} OA: Cu^+_{(aq)} + e^- \to Cu_{(s)} \\RA: Cu^+_{(aq)} \to Cu^{2+}_{(aq)} + e^- \\2Cu^+_{(aq)} \rightleftharpoons Cu_{(s)} +Cu_{(aq)} ^{2+} \end{gather}
Combinations (elaboration)
Constructing Redox Half Reactions
…
- Write two half reactions using the given information and pair the same elements together.
- Balance all atoms except for hydrogen and oxygen.
- Add water to balance the oxygen (on the opposite side of ).
- Add ions to balance the hydrogen (on the opposite side of the water/same side as ).
- Balance the total charges on both sides by adding . (The side with tends to get the ).
- ‘Clean up’ and write the net ionic equation = final balancing so species cancel out.
Example 6
Balance the following redox reaction. And label the SRA and SOA:
Oxidation numbers
Oxidation numbers are a way to keep track of and account for electrons in a redox reaction. This number describes an atom’s oxidation state. Where an oxidation state is the apparent electric charge of an atom (Ions have an oxidation number equivalent to their charge. If this ion is polyatomic, the sum of the apparent charges within the ion must add up to the overall ionic charge).
| Chemical | Oxidation number | Examples |
|---|---|---|
| Remaining elements | 0 | Na(s) Cl2(g) S8(s) |
| Hydrogen | +1 | HCl, NaOH, CH3COOH |
| (^exception=Hydride) | -1 | LiH, MgH2 |
| Oxygen | -2 | H2O, CaO |
| (^exception=Peroxides | -1 | H2O2 - H = -1 |
| Monatomic ions | ionic charge | Na+ = 1+; Ca2+ = 2+ |
The other atoms in the formula must now receive a number that allows all the oxidation numbers to add up to the overall charge. Numbers are assigned individually, but add up/multiply with the coefficients to reach to overall charge.
Balancing with oxidation numbers
Given a net ionic equation, oxidation numbers can be used to deduce whether something is oxidized or reduced. If the oxidation numbers increases, it loses electrons; is being oxidized and is an RA, the inverse is true if the oxidation number decreases.
Spontaneity !!
Solving for Cell Potential
If is negative, no reaction occurs. If is positive, it is spontaneous and a reaction occurs. If , the cell is at equilibrium and non-spontaneous.
Gibbs Free Energy
Where is the moles of electrons transferred, is the charge of one mole of , .
Spontaneity of a Cell
If the then and therefore spontaneous. If the then and therefore non-spontaneous. If the then and therefor at equilibrium. If the then and therefore at equilibrium.
Nernst Equation (non-standard conditions) !!
Voltaic Cells
Definitions:
- Electrochemical cells: Convert chemical and electrical energy back and forth
- Voltaic/galvanic cells: Converts chemical energy into electrical energy; Multiple voltaic cells combined to form a battery
- Electrolytic cells: Convert electrical energy into chemical energy; Rechargeable batteries combine both a voltaic and electrolytic cell.
Voltaic Cell Components:
- Voltaic cells are composed of two electrodes a positive end (a cathode) and a negative end (anode). Wherein electrons flow from anode to cathode.
- Two solutions: one at each solution.
- Salt bridge/semi-permeable membrane. This is done to ‘connect’ the two solutions and prevent charge accumulation.
- A wire: to allow electrons to flow through.
- Voltage (Volts, V): Is the electric potential difference. And is dependent on the materials that compose the cell. “How strong is the movement of the electrons”
- Current (Amperes, A): The amount of electric charge moving around in an electric circuit.
- Charge, (Coulombs, C): The amount of charge (electrons or lack of) that are transferred.
Electrolytic Cells
Two half-cells that are separated by a physical boundary. The components of the two half cells undergo a redox reaction spontaneously. A voltaic cell allows the electron transfer of the redox reaction to occur over the wire.
A single line - represents phase boundary (e.g. solid vs. aqueous); A double line - represents a physical boundary.
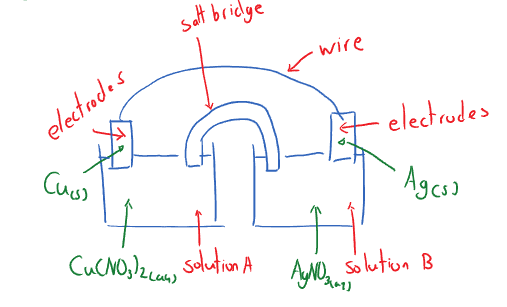
In the diagram above there are two half cells that are separated by a physical boundary. The components of the two half cells undergo a redox reaction spontaneously. This is done such that voltaic cell allows the electron transfer of the redox reaction occurs over the wire.
Anodes
The SRA is always being oxidized at the anode. Over time, the anode loses mass, and the concentration of the solution its in solution increases.
Cathodes
The SOA is always reduced at the cathode. This can be memorized using the pnemonic: ‘red cat.’ In addition, the cathode gains mass due to the deposition of of the solid product. The ionic concentration within the solution its in decreases.
Salt Bridge
The salt bridge allows the solutions to remain electrically neutral. As overtime, the anode will become increasingly positive and the cathode increasingly negative. The salt bridge is effectively completing the circuit. Such that the electrolytes in the solution can balance the charge.
- Any positive ions will move through the salt bridge towards the cathode side to balance charge.
- Any negative ions will move through the salt bridge towards the anode side to balance charge.
Notation
Anode | electrolyte || electrolyte | cathode (This is how it is supposed to be formatted; on the diploma and on certain questions this will be out of order.)
Inert electrodes:
For a voltaic cell, the cathode is not a participant in the reaction. Inert electrodes - in particular, because the cathode does not participate in the reaction it can be replaced with another conductive material. Ensure that this does not undergo a reaction with the solutions. Inert electrode examples: Platinum, but something better, graphite.
Electric Potential
The voltage is a measure of the ability of electrons to do work. Wherein an increase in voltage increases the potential or capacity for the electrons to do work.
Cell Potential
A standard cell is a voltaic cell where all the entities are at . The standard cell potential, is calculated by taking the difference in potential between the cathode and the anode. (In the data booklet, all reduction half reactions are relative to ). To get the voltage for the oxidation number, multiply the voltage for the half reaction by . If calculating the cell potential using the oxidation value, it must be added to the reduction half reaction. As such there are two formulas
Solving for cell potential
- Determine the SOA and SRA
- Write the reduction half reaction and the reduction potential (SOA)
- Write the oxidation half reaction and flip the value of the reduction half reaction potential (SRA)
- Balance the half reactions and add the reduction potential and
Electrolysis
Electrolytic cells
Whereas a voltaic cell uses a spontaneous chemical reaction to convert chemical energy to electrical energy, an electrolytic cell, a non-spontaneous reaction, is forced to occur when supplied with electricity. This process ‘give’s’ a reaction the potential to perform the reverse reaction spontaneously (how batteries can be recharged for example.)
Components
- Two electrodes (a cathode and an anode)
- One electrolytic solution
- An external power source, such as a battery
Comparison to a voltaic cell
- Non-spontaneous reactions (OA will be ‘lower’ than the RA)
- The cell potential is negative (this describes how much voltage is needed to run the cell)
Cathode
The cathode is still the site of reduction, but is now is considered to be negative. Such that the electron flow still goes from anode to cathode. The cathode can be inert similar to a voltaic cell because the reaction is happening in the solution.
Anode
The anode is still the site of oxidation, but is now considered to be positive. Such that the electron flow goes from anode to cathode. The anode can be inert as well as the reaction is happening in the solution.
Notation:
Corrosion
Corrosion, also known as rusting and reduction of a metal is a common occurrence in as air is composed of a common oxidizing agent, . Which is only catalyzed in the presence of and salts.
Aluminum corrosion
. This can be qualitatively be observed as aluminum oxide is ‘sticky’ and adheres to the outer layer of solid aluminum. Therefor the aluminum underneath does not undergo further oxidation.
Iron corrosion
As iron and its derivative alloys are often used for construction, its corrosion must be maintained. . As iron oxides do not adhere to iron, it flakes off and often forms . A fresh layer is exposed which causes further oxidation and flaking.
Corrosion protection
Corrosion is an issue that needs to be controlled and managed. In particular can be worse in areas with more moisture and salt. This effects costal cities and cities with lots of participation the most. What cities do to protect themselves against this includes:
- A coating: e.g. paint, epoxy/plastic coating, electroplating and grease/fat.
- Pros: Cheap, quick to apply, effective
- Cons: It can chip and wear out which would require constant maintenance → which can be expensive.
- Cathodic protection
- Corrosion always occurs at the anode where the reducing agent is (the metal)
- If the anode is turned into a cathode, we can stop this from spontaneously reacting
- Adding a current does this as the way electrons are flowing from anode to cathode, the negative terminal of the battery and hook it to the metal (thereby forcing electrons into the metal)
- Sacrificial anode/metal = placing a small amount of a better reducing agent (such as zinc and magnesium) which allows for the redox reaction to occur with the sacrificial metal before the protected metal.
- Galvanizing = A sacrificial zinc coating is put onto the surface of the protected metal through electroplating.
- All methods are combined for the best result.
Application of electrolysis:
It is used in the production of pure elements and to refine materials. Additionally it is used in electroplating.
Pure materials (water example)
In the electrolysis of water, water is electrolyzed into and : \begin{gather}RA: 2H_2O_{(l)} \to O_{2(g)} + 4H^+_{(aq)} +4e^- \\ OA: (2H_2O_{(l)} +2e^- \to H_{2(g)} + O_{2(g)} \\ 6H_2O \to O_{2(g)} + H_{2(g)} + (4H^+_{(aq)} + 4OH^-_{(aq)} = 4H_2O_{(aq)}) \\ 2H_2O_{(l)} \to O_{2(g)} + 2H_{2(g)} \end{gather}
Refinement of materials (purification of metal ores)
When metals are mined, they are often contaminated with dirt and other impurities. With electrolytic cells, these metals can be purified.
Cell Stoichiometry
This allows us to relate the number of electrons transferred with mass gained and lost. The amount of electricity passed has a direct relationship with mass.
Where = amount of electric charge, (Coulombs, C), = current (Amperes, Amps, A), = time (seconds, s). has to be converted into the actual amount of electrons. This is done by multiplying Avogadro’s ratio of particles per mole, by the charge of an electron, = Faraday’s constant, . When substituted into , the following formula for the amount of moles of an electron is:
Chloride Anomaly
During electrolysis, the predicted reaction for a ion acting as a weaker RA than is false. As when chloride ‘competes to be a stronger RA with water, it acts as a stronger RA.
Cathode: , Anode: Net reaction: