2024-07-1213:41 Status:IBnotes Tags: Atomic physics Chemistry
General model.
Context
Democritus, 460-370 BCE, was the first to use the term, atom to describe small indivisible particles which composed all matter. This was overshadowed by Aristotle’s fire-earth-air-water model, and prevailed through alchemy. That is until John Dalton (1766-1844) described elements and chemical reactions in terms of atomic theory. He proposed that each element was made from a unique atom. (Coming up with a symbol system that isn’t used to day)
Models of the Atom - Abridged
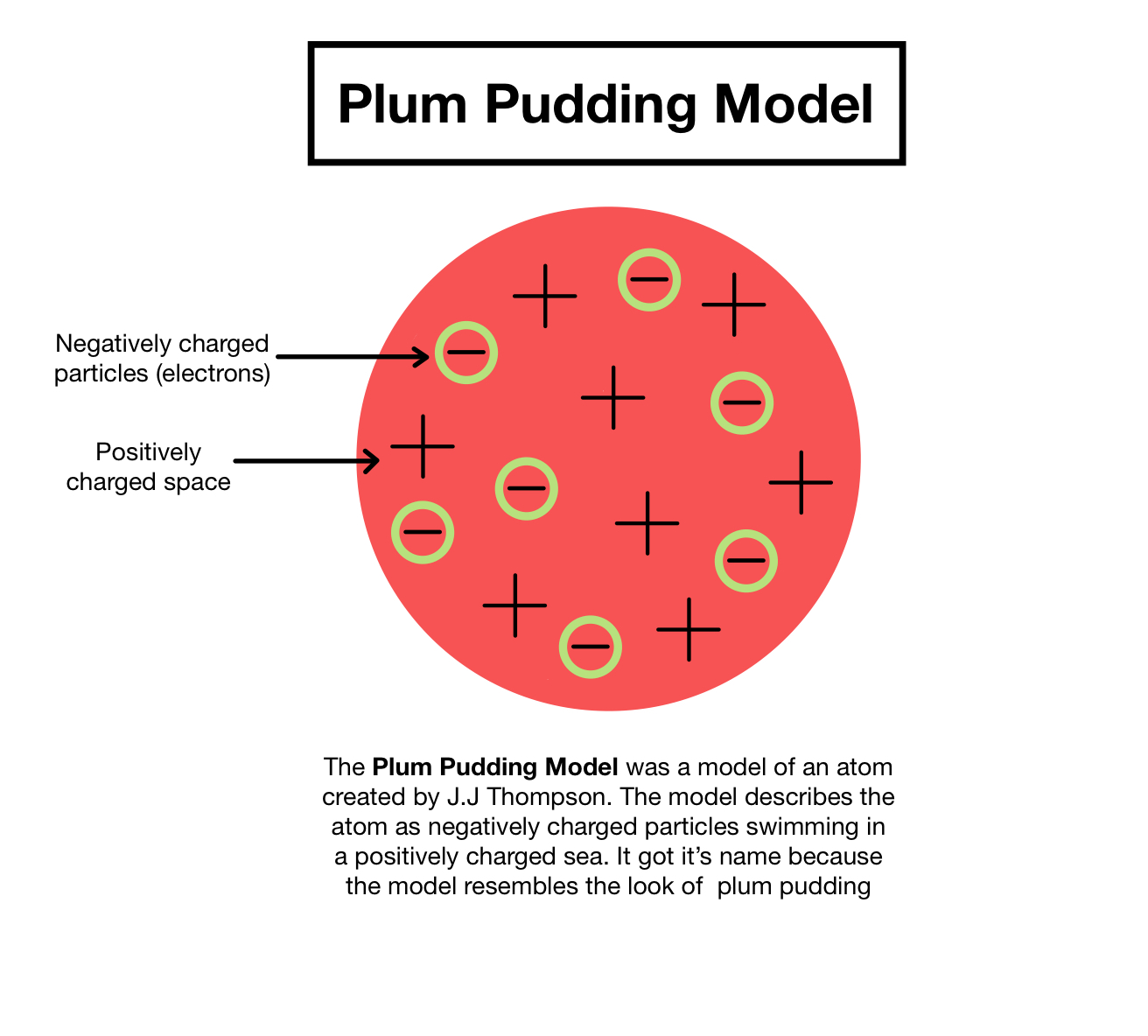
Electrons were the first subatomic particle to be identified by charge by J.J. Thomson in 1899. Thomson devised a model of the atom, referred to as the Plum Pudding Model. In which negative particles were dotted throughout a mass of positive charge. This model was disproved by Rutherford’s Golden Leaf experiment in 1919. In which Rutherford discovered protons and that the nucleus was composed of small dense protons and neutrons. 20 years later, the invention of the mass spectrometer explained the true nature of isotopes. In 1932m James Chadwick identified the neutron.
Models of the Atom - Unabridged
Advent Dalton: (not curriculum)
Atomism = a discussion of the smallest unit of indivisible matter
Dalton
Dalton proposed that atoms were like tiny, solid, billiard balls, which could join together in small whole-numbered combinations to produce various compounds. This model still explains the aspects of chemistry well - although it lacked the inclusion of charges.
J.J. Thompson
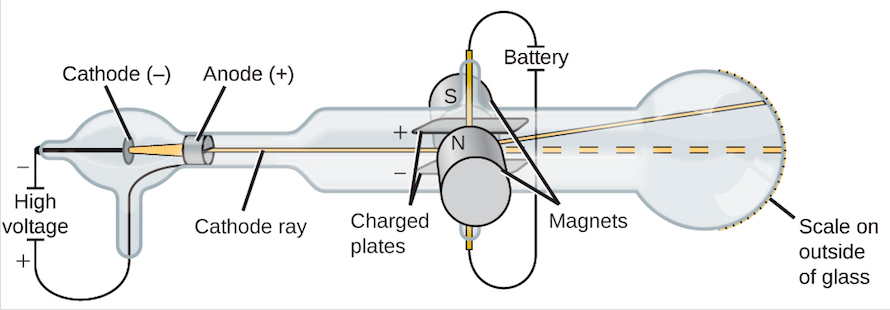
J.J. Thompson used cathode ray tubes to deduce that there was some property of an atom that had to retain a charge. The cathode ray tube used was a sealed tube that was evacuated from air. A high voltage source is used to eject high energy electrons from the cathode (negative electrode) to the anode (positive electrode). A metal plate, coated with phosphors that emit green light when struck by electrons, is positioned inside the tube to detect the path of electrons. The properties of Cathode Ray Tubes, CR(T)s are as follows
- If an object is placed in the path of a CR, a shadow of the object is cast on the glowing tube wall at the end. This showed that the CR travelled in straight lines.
- The CR can push a small paddle wheel up an incline against the force of gravity. This showed that the CR carried energy and could do work.
- The CR can produce chemical reactions similar to light (i.e. expose photographic film (also known as (X-Ray film badges))
- The CR is deflected from a straight line path by a magnetic field, suggesting that the two were related in some way.
- CR were experimentally shown to be negatively charged in 1885
- J.J. Thomson is the first person to succeed in deflecting the CR with an electrical field in 1897. He bent them towards the positive pole which confirmed that the cathode rays were negatively charged.
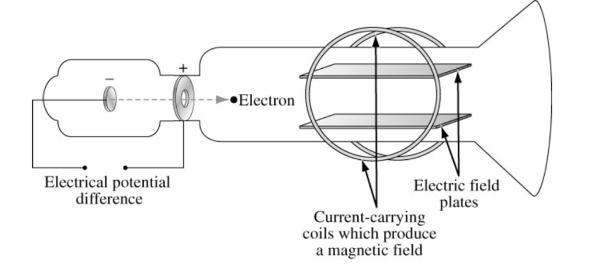
Thompson’s Cathode ray experiment is as follows:
As in the diagram below, the two plates about midway in the CRT were connected to a powerful electric battery, thereby creating a strong electrical field through which the cathode rays passed. Thomson also could use magnets which were placed on either side of the straight portion of the tube just to the right of the magnetic plates. This allowed him to use either electrical or magnetic or a combination of the both to cause the cathode ray to bend. The amount the cathode ray bent from the straight line using either the electric field or the magnetic field allowed Thomson to calculate the charge to mass ratio, .
This was done as follows: \begin{gather} \frac {mv^2} r = qvB \newline \frac q m = \frac {v}{rB} \newline \frac q m \approx 1.76*10^{11} C/kg \end{gather}
Where is the charge mass ratio, is the selected velocity of the particle, is the radius subtended by the arc created by the curving particle and is the magnitude of the magnetic field strength.
His model of the atom is known as the Raisin Bun/Plum Pudding model of the atom. Thomson found all CR had the same charge to mass ratio regardless of the metal used in the cathode or the accelerating voltage. This lead Thompson to conclude that the CRs came from a fundamental particle within each atom. Because particles originated at the cathode, these particles must be negatively charged. He therefore proposed a model of the atom as a large positively charge mass containing small negative charges embedded within/on the surface of the atom. These negatively charged particles were named electrons.
Thomson was the first to explain the electrostatic nature of the atom.
The relationship between the radius of curvature of cathode rats and accelerating voltage is derived as follows:
\begin{gather} F=qvB \newline qvB=\frac {mv^2} r \newline \frac q m = \frac v {Br} \newline \frac 1 2 mv^2 =qV_{accelerating} \newline v = \sqrt {\frac {2qV_{accelerating}} m} \newline \frac q m =\frac {2V_{accelerating}} {B^2 R^2} \end{gather}
Rutherford
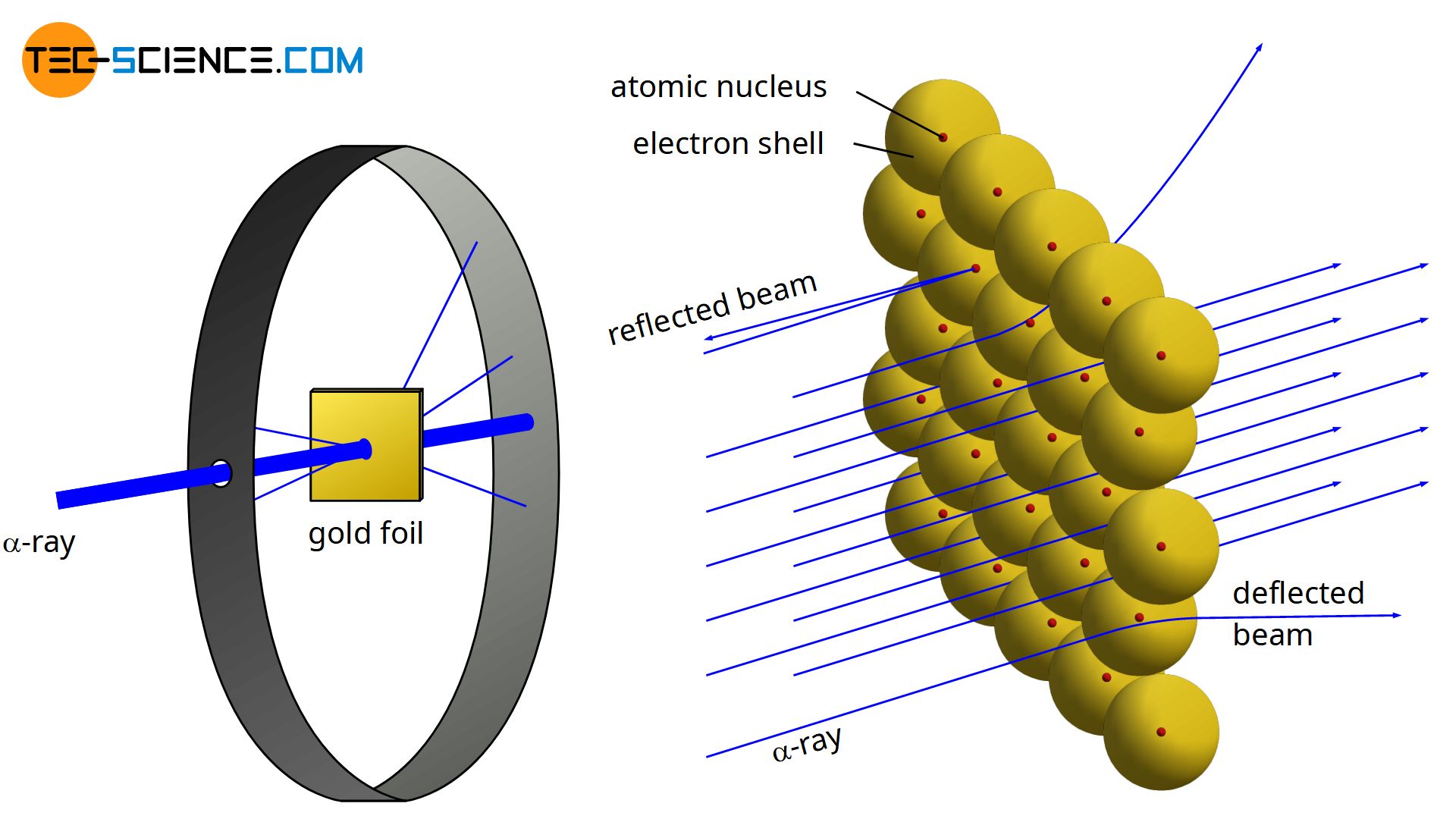
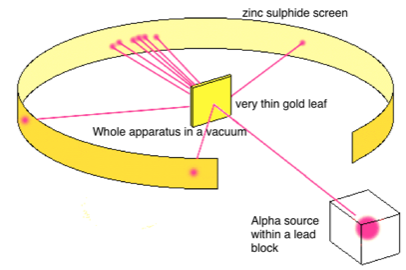
Rutherford’s model was created as a result of discovering that the majority of the atom is empty space. In his experiment, he found that when he bombarded a thin m thick gold foil, the majority of alpha particles would pass through. They were emitted from radioactive plutonium and detected with a zinc sulfide film (which would glow when struck) He observed the following in his experiment
- Most alpha particles pass straight through the gold foil
- Some of the alpha particles get deflected by very small amounts
- Ver few were deflected greatly
The conclusions that he drew in light of this were:
- The atom is 99.99% empty space
- The nucleus contains a positive charge and most of the mass of the atom
- The nucleus is approximately 100,000 times smaller than the atom
Bohr
Bohr’s model of the atom was required because if Rutherford’s model were to be correct, electrons would orbit the nucleus to counteract the electrostatic force of attraction between the nucleus and electrons. According to Maxwell, the electron should constantly radiate energy and spiral into the nucleus (which it does not do so). Additionally, when gases are electrically excited only specific spectral lines are emitted. According to Rutherford, the electrons should be able to orbit at all possible radii and therefore emit all frequencies of light.
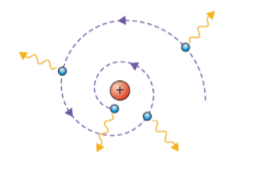
Bohr’s model of the atom states that
- Of all the possible circular and elliptical orbits around the nucleus there were only a few allowed orbits. Each requiring a different energy level. These were the energy states of the atom.
- When moving in an allowed orbit, the atom does not radiate EMR
- Electrons may jump from a higher energy level to a lower one. When the electron makes such a transition, the energy difference of the two orbits is given off as a single photon. Similarly, energy equal to the difference in energy between a lower state and some higher state. Bohr had trouble explaining why what he claimed was true
De Broglie explained Bohr’s orbital radii for hydrogen by incorporating wave nature of electrons. He showed the orbital radii calculated by Bohr corresponded to the standing wave patterns of electrons around the nucleus. A standing wave is only possible if a discrete number of electron wavelengths fit exactly along the circumference of an orbit. — This explained why only certain stationary states were present. When an atom is in an excited state (above E_1), then it will only remain in that state a short time before dropping to a lower energy level. When the atom drops a photon of EMR is given off equal to the energy change of the atom.
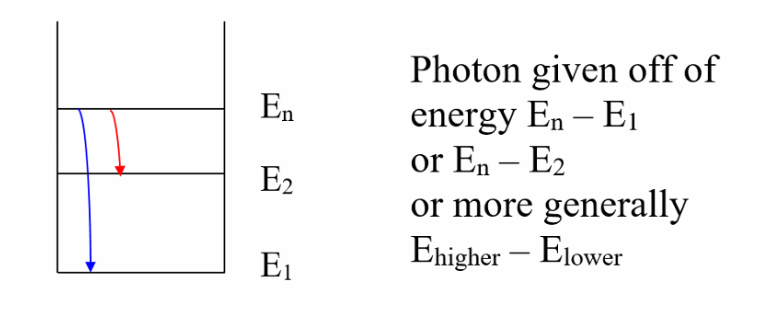
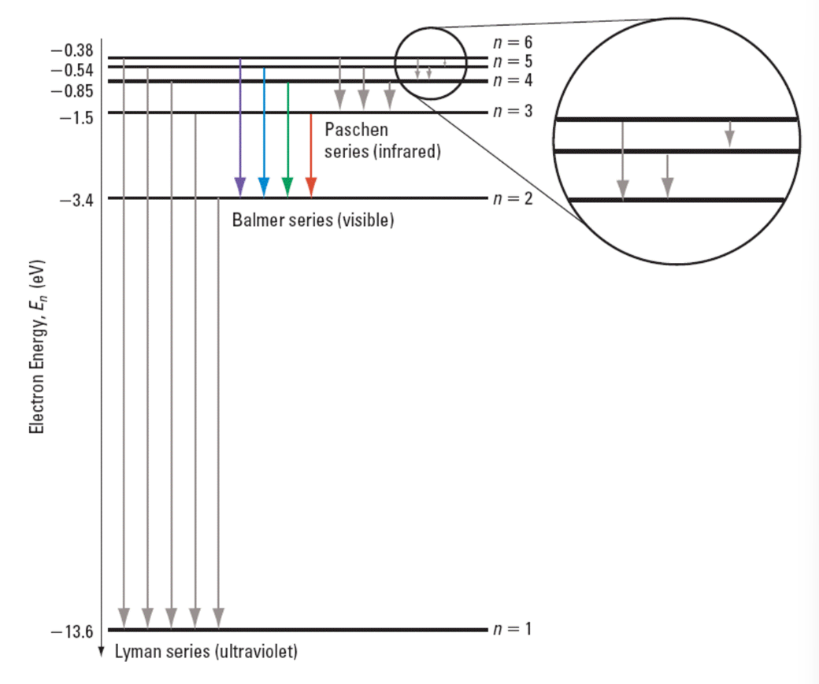
The photon is absorbed if the energy level of the atom increases, and emitted if it goes back down. Additionally if the incident photon energy dos not exactly match an energy level change it will not be absorbed at all. The absorption spectrum is when light frequencies exactly matching an energy level change a lower energy level to a higher energy level. These photons are absorbed by the atom and re-emitted in every possible direction (may look like the photon goes undedicated by it may have been deflected in a specific manner). Most of the photons go off in a different direction that the original beam of light therefore there appears to be sections of the spectrum that are darker than usual.
Quantum model of the atom/Schrödinger
The quantum mechanical model of the atom is a mathematical model based on statistical probabilities of energy levels within various atoms. Quantum mechanics works for all atoms not just the hydrogen atom. This is an evolving model that incorporated whatever new discoveries are made. Quantum mechanics
TLDR:
- Dalton = Billiard ball model
- JJ Thompson = Plum and Pudding/Raisin Bun model and ratio was constant (Dalton but with electrons)
- Rutherford = Solar System model + mostly empty space, the majority of the mass of the atom was within the nucleus. The nucleus was positively charged
- Bohr solved rutherfords model - energy levels + emission but had trouble explaining why
- ….
Helium neon laser: Helium gas atoms get excited to energy level due to collisions with electrons in the tube. These Helium atoms randomly collide with neon atoms causing Helium to de-excite and neon to move to the level which is metastable (atoms can be metastable for approx. s compared to the typical s). To start lasing action one atom often drops from to . This photon triggers other atoms to also drop by the same amount of energy.
The Atom
When Niels Bohr was working with Rutherford in 1913, he proposed a model of the atom that explained emission spectra that had been discovered. Bohr’s model proposed that electrons moved around the nucleus in shells, which were regions of space with fixed energies. About 100 years later, Bohr’s model is still acceptable as a model that explains the atom, although more accurate models exist.
| Subatomic particle | Symbol | Mass kg | Relative mass | Charge, C | Relative charge | Position in the atom |
|---|---|---|---|---|---|---|
| Proton | p+, p | 1.6726 x 10 ^-27 | 1 | +1.6022 x 10^-19 | +1 | In the nucleus |
| Neutron | n0, n | 1.6749 x 10 ^-27 | 1 | 0 | 0 | In the nucleus |
| Electron | e-, e | 9.1064 x 10 ^-31 | 1/1836 | -1.6022 x 10^-19 | -1 | Orbiting the nucleus |
Masses and relative masses
Protons and neutrons have approximately the same mass, with the neutron being very slightly heavier than the proton. Given that their actual mass is very small, it is simpler to use relative masses. Protons and neutrons have the relative mass of 1, whereas the electron, having a significantly smaller mass, aren’t included in the mass of the atom and have a ‘relative mass’ of 0.
Charge
Electrons have a charge negative but equal in magnitude to the charge to that of the proton, being 1.6022 x 10^-19. This is the elementary charge, e. Additionally, neutrons have no charge.
Configuration
Protons and neutrons make up the nucleus of the atom; where the majority of the mass of the atom is. The electrons are found in ‘shells’ around the nucleus.
In a neutral atom, there is an equal amount of protons and electrons. (Leptons, Gluons and other particles will not be discussed in great detail in this course)
Atomic number, mass number and isotopes
The atomic number, represented by the symbol , is the number of protons in the nucleus. It is also referred to as the proton number. The amount of protons within the nucleus of an atom is what distinguishes it from another element.
The mass number, represented by the symbol , is the sum of the number of protons and neutrons and protons. (mass number = + number of neutrons)
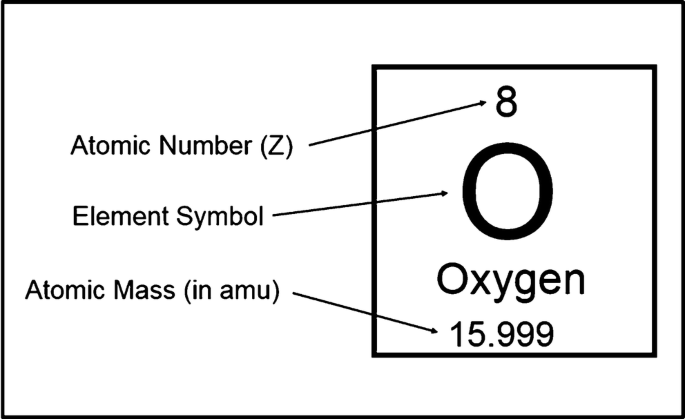
Isotopes
Isotopes are elements that have the same number of protons, but a different amount of neutrons. To represent this, nuclide notation is used. Nuclide notation combines mass number, atomic number and symbol, as seen with oxygen above. Alternatively the atomic number and symbol are replaced with the name of the element followed by it atomic mass. For instance, would be represented as silver-107
Isotope properties
The chemical properties of atoms are determined by their electronic structure; however, their physical properties depend largely on their nuclei. This entails, the physical properties of an atom are different to one of it isotopes despite having the same or very similar chemical properties. One example of this is the differing masses of isotopes such as ‘heavy water’, which is denser than , and take up 11% less volume. Other physical properties that can change include but are not limited to, boiling point, melting point, and the rate of diffusion.
Other differences are more complicated for instance:
- Several forms of spectroscopy rely on the unique nuclear properties of specific isotopes.
- Nuclear magnetic resonance, NMR spectroscopy can be used only for isotopes with a non-0 nuclear spin. and are both isotopes that have non-0 nuclear spins and are can be used in NMR spectroscopy. More on this in Option A.
- Many isotopes, are radioactive -referred to as radioisotopes- and have their respective applications.
- In decaying organisms, exists in a set ratio with . When an organism dies, carbon-14 decays and carbon-12 does not. The percentage decrease as the age of the dead organism increases with the age. This is used to estimate the age of the organism. This is radiocarbon dating
There are also some dangers that come with radiation (more in Atomic physics) + Radioactive poisoning is the term used to refer to the acute problems caused by large doses of radiation from radioisotopes in a short period. Large amount of radiation interfere with cell division, and this results in many of the symptoms of radiation poisoning
The discovery of radiation is attributed to German scientist Wilhelm Roentgen in 1895. Roentgen was using a cathode tube covered in black paper when he noticed a screen on the other side of a darkened room fluorescing. Some invisible rays must have been passing from the tube to the screen. Roentgen named the rays X-rays; he even thought to X-ray his wife’s hand. Medical science ran with this new idea in a big way. A year later, Henri Becquerel, a French scientist, found that materials such as uranium emit X-rays. Marie Curie and her husband Pierre found that the ore pitchblende is even more radioactive than uranium. Curie isolated the elements polonium and radium from this ore. Marie Curie died of leukaemia, believed to have been caused by prolonged exposure to radiation during her research work. We now know that a radioactive element decays. This means that the nucleus is unstable and it ejects small particles. The particles ejected have been labelled alpha, beta and gamma particles. Alpha particles are helium nuclei, beta particles are electrons and gamma particles are a stream of photons. When alpha particles are ejected from the nucleus a new element is formed.
| Radioisotope | Use |
|---|---|
| Carbon-14 | Radiocarbon dating. The ratio of carbon-12 to carbon-14 is calculated to determine the age of an deceased organism or an object with carbon. |
| Iodine-131 | As a medical tracer in the treatment of thyroid disorders. The radioactive iodine is taken up by the thyroid gland and then the radiation kills part of it. |
| Iodine-125 | As a medical tracer in the treatment of prostate cancer and brain tumours. It also is taken up by the thyroid gland. |
| Cobalt-60 | Radiotherapy, levelling devices and to sterilize foods and spices. |
| Americium-241 | Smoke detectors. Emits a beam of alpha particles which, if interrupted by smoke, will set the device off |
| Technetium-99 | Radiotherapy for cancer and for studying metabolic processes. Emits low energy radiation, so small doses can be administered. |
| Medical tracers, radioactive forms of atoms, can be attached to molecules that target specific tissues in the body, such as cancerous tissue. The isotopes of iodine listed above are both examples of medical tracers. |
Ions
Ions are atoms that have lost or gained electrons now have a net charge. A positive ion, a cation, has fewer electrons than electrons; inversely, a negative ion, an anion has more electrons than protons.
The Mass Spectrometer
A mass spectrometer is an instrument that accelerates charged particles through a magnetic field. The degree to which the particles are deflected from their original path will be dependant on their charge to mass ratio; m/z ratio. The process in which this is determined is done in the following main stages:
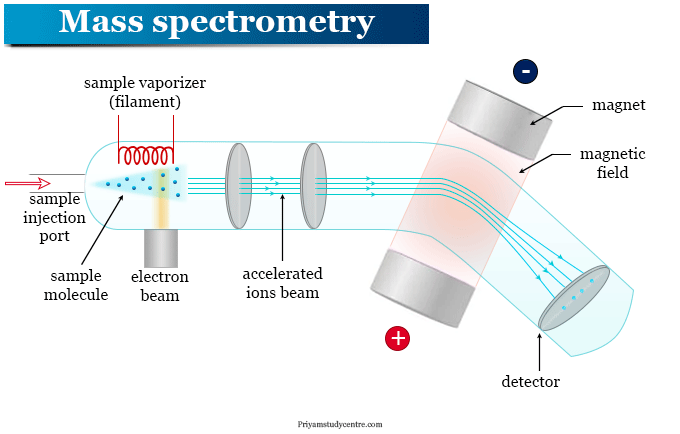
- Vaporization: The sample to be analyzed is heated and vaporized and then passed into an evacuated tube. This results in the ‘filtering’ of particles.
- Ionization: The atoms or molecules are then bombarded by a stream of high energy electrons and one or more electrons are knocked off each atom or molecule. This results in ions, which most commonly, a 1+ charge, but sometimes a +2 charge.
- Acceleration: The positively charged ions are accelerated along the tube by attraction to negatively charged plated and the ions pass through slits that control the direction and velocity of their motion.
- Deflection: The stream of ions is passed into a strong magnetic field, which deflects the ions through a curved path. If the side of the magnetic field is fixed, a light ion will e deflected more than a heavy ion and a 2+ ion will be deflected more than a 1+ ion with the same mass. The deflection of the ions depends on the m/z ratio. In modern mass spectrometers the strength of the field is variable.
- Detection: The ions are detected electronically by a device that measures the location and the number of particles that collide with it.
-
- Recording: The percentage abundance, of the different isotopes is recorded as a graph called a mass spectrum. A peak is produced in the mass spectrum for each isotope (ion with a particular mass and charge). The position of the peaks along the horizontal axis indicates the mass/charge ratio.
.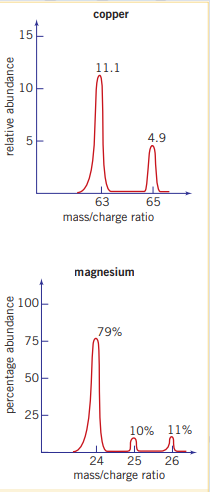
In simple elemental mass spectra (ions only have single charges), the number of peaks recorded indicates the number of isotopes of the element there are. The height of each peak speaks to their respective frequency
To generate the relative scale of atomic masses, chemists chose the most abundant isotope of the element carbon, carbon-12 and assigned it a relative mass of exactly 12 units. It was chosen for the following reasons:
- Carbon is cheap and widely available
- It is relatively easy to isolate and purify this isotope
- carbon is not toxic in any way
It was decided to assign carbon a mass of 12 units as this number was indicative of the mass number of this isotope. using a mass spectrometer, the lightest of all the elements was found to deflect 12 times further than the standard carbon-12 isotope, and the most common isotope of magnesium, magnesium-24 was deflected half as far as carbon-12. Thus hydrogen, the lightest element, have a relative mass of 1 and magnesium-24 a relative mass of 24.
Calculating relative atomic mass
The formal definition of relative atomic mass is useful in helping to recall how to mathematically determine its value for a particular element.
The relative atomic mass, RAM of an element is defined as the weighted mean of the masses of its naturally occurring isotopes on a scale in which the mass of an atom of the carbon-12 isotope is 12 units exactly. The symbol for RAM is .
To determine the of an element, X, multiply the relative isotopic mass, (RIM, ) of each naturally occurring isotope by its abundance faction and then add all those values. This can be represented mathematically as follows:
\begin{gather} A_r(X) = \Sigma (I_r \times abunadance⠀fraction) \newline ⠀ \newline A_r(X) = \frac{\Sigma (I_r \times abunadance⠀fraction⠀expressed ⠀as⠀a⠀percentage)}{100} \end{gather}
Electron Arrangement
Electronic Configuration
There is a specific order in which electrons in core electrons
The Electromagnetic Spectrum:
Light consists of electromagnetic waves, where the wavelength, is the distance between two crests and ranges from approximately for red light and . The frequencies of which are and for red light and violet light respectively. This can be mathematically determined using the following formula.Where is the energy of the light/photon particle, is the wavelength, is Planck’s constant, is the speed of light. This can be rearranged to use frequency, f (sometimes ), using the following formula:
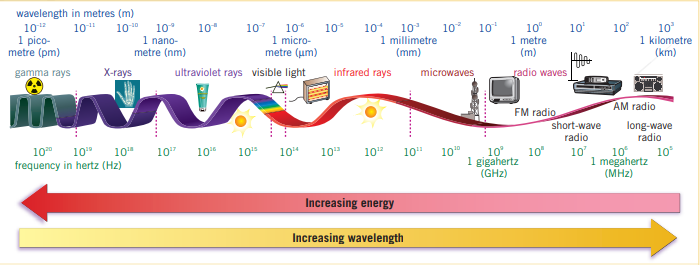 As energy increases, the frequency of the light also increases. Thus red light is of lower energy to violet light. High energy is often more dangerous than low energy rays. Visible light is part of a much large spectrum of light as seen above.
As energy increases, the frequency of the light also increases. Thus red light is of lower energy to violet light. High energy is often more dangerous than low energy rays. Visible light is part of a much large spectrum of light as seen above.
When sunlight or white light is incident on a prism, the component wavelengths are bent or refracted at different angles. This produces a continuous spectrum, similar to how when sunlight passes through raindrops and produces a rainbow.
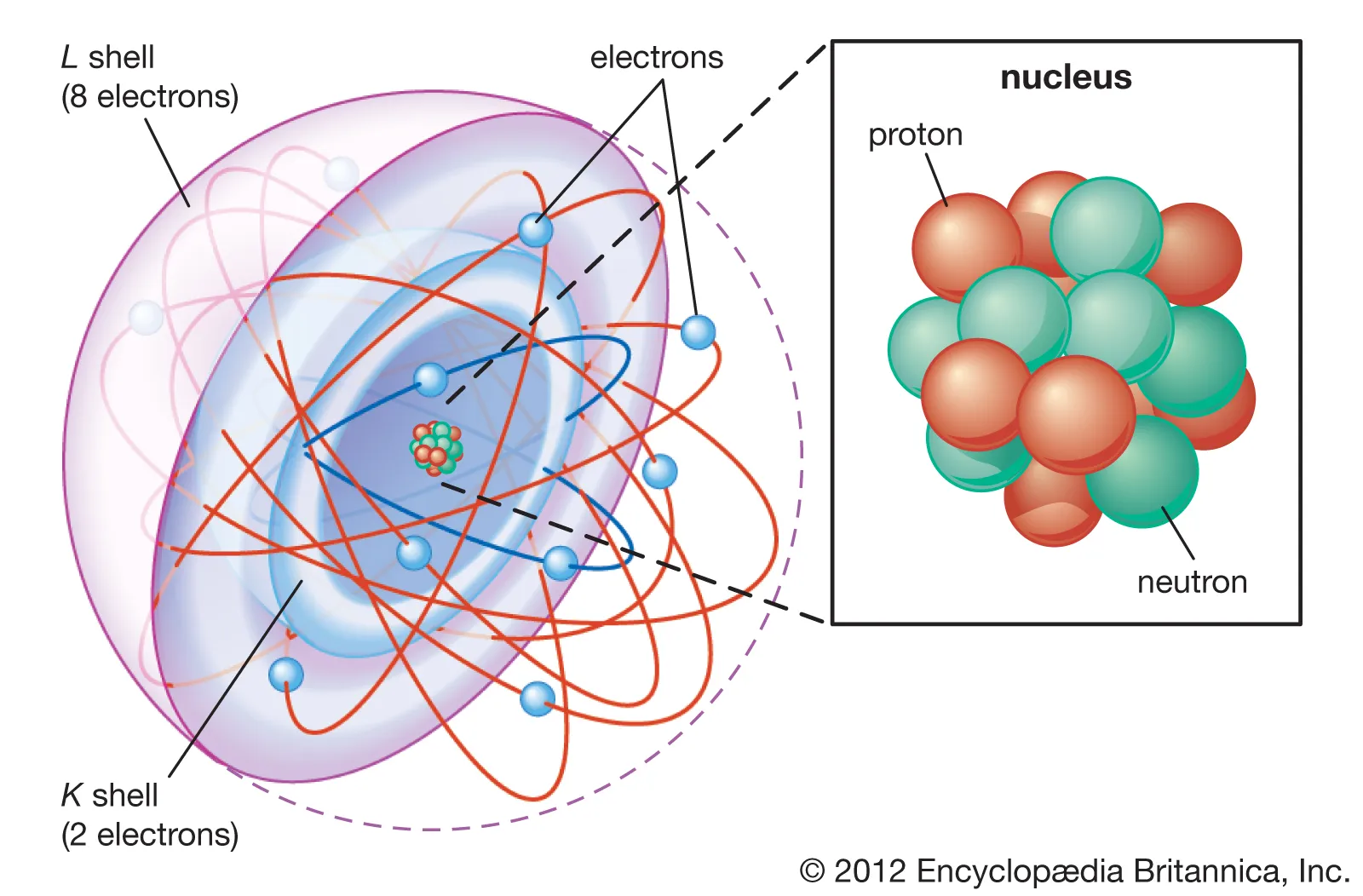
The Bohr Model of the Atom
In 1913, Niels Bohr proposed that the electrons move around the nucleus with a fixed amount of energy in shells. He proposed that each atom has a series of these shells. The shells close to the nucleus are of low energy and those further out are of higher energy. Shells are numbered outwards from the nucleus; 1,2,3 … and are also identified by letters similarly; K,L,M … . Electrons move around the nucleus in these shells in pathways referred to as orbits.
According to Bohr’s model of the atom, different shells hold differing numbers of electrons. The maximum number of electrons that can fit in a given shell can be modeled by the equation, , where is the shell number.
If an atom had 11 electrons for instance, its electron arrangement would be 2,8,1 with 1 valance electron. As all electrons are as close as possible to the nucleus, it is in its lowest energy state, the ground state.
| Shell number, n | Maximum number of electrons in this shell, 2n^2 |
|---|---|
| 1 | 2 |
| 2 | 8 |
| 3 | 18 |
| 4 | 32 |
| Element name | Atomic number | Symbol | Charge | Electron arrangement |
|---|---|---|---|---|
| Nitrogen | 7 | N | 0 | 2,5 |
| Oxygen | 8 | O | 0 | 2,6 |
| Neon | 10 | Ne | 0 | 2,8 |
| Chlorine | 17 | Cl | 0 | 2,8,7 |
| Nitride ion | 7 | N 3- | 3- | 2,8 |
| Oxide ion | 8 | O 2- | 2- | 2,8 |
| Sodium ion | 11 | Na + | 1+ | 2,8 |
| Calcium ion | 20 | Ca 2+ | 2+ | 2,8,8 |
Evidence for the Bohr model: Line spectra
Experimental evidence for Bohr’s model came from studies of the emission spectra of atoms. The spectra were emissions of light from atoms that have been provided with energy such as heat, light or electricity. The bright colour of fireworks are an example of such emissions.
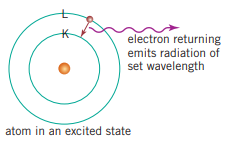
Bohr explained emission spectra by suggesting that if atoms were subjected to large amounts of energy from heat, light or electricity, the electrons would can change their energy levels. The electrons jump to an energy level further than the one that they would normally occupy. The electrons make specific jumps depending on the energy given to each electron. The atom is said to be excited when this happens. When the electrons return to their ground state, the extra energy is converted into light and emitted at a specific frequency, dependant on the difference of energy between the ground state and the excited state. The emitted light, a line (or emission) spectrum, looks like a series of coloured lines on a black background. Some of the emissions may be radiation of a wavelength that is not visible to the naked eye. The study of this light emitted from the atom is called emission spectroscopy.
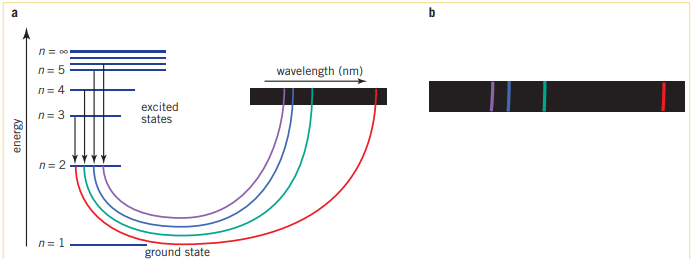
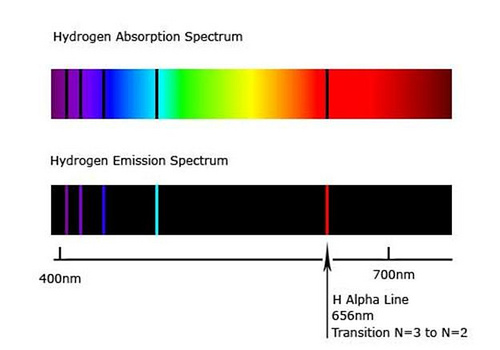
Based off of the energy difference, the wavelengths emitted would be of different magnitudes, and only some visible. The spectrum that the human eye can see, the Balmer series has excited electrons from and to the 2nd energy level. The higher energy, ‘invisible’, Lyman series has excited electrons from and to the 1st energy level. Finally the lower energy ‘invisible’, Paschen series has electrons coming from and to the 3rd energy level. (This is where the energy level mentioned is the lowest energy involved withing the energy transition)
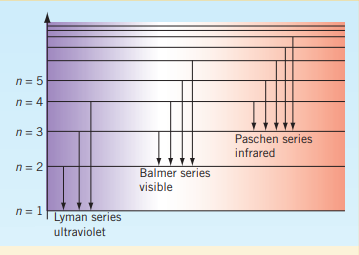
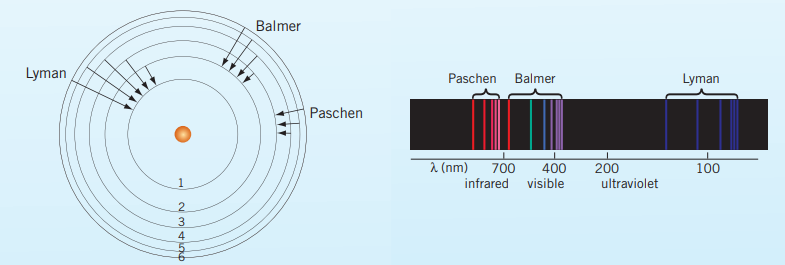
Bohr’s model of the atom explained the closeness of the emission lines in terms of the decreasing difference between the energies of the shells as their distance from the nucleus increase. The lines became closer together as their energy increase because the energy of the shells is increasing by diminishing amounts. As this progresses with the increasing amount of protons and hence electrons, the emission spectrum appear to form a spectrum. This phenomenon of ‘merging’ into a continuum is the convergence of the emission spectrum.