2024-07-1616:19 Status:IBnotes Tags: Thermodynamics
4.1 The Mole Concept and Avogadro’s Constant
A mole is defined as the amount of particles required for carbon-12 to have a mass of 12g exactly. This comes out as being particles, Avogadro’s constant, L. - Where having 1 mole of carbon is similar to having one dozen eggs, but defined by using carbon as a reference point. Because of this, a mole is a relative quantity. It is because of the composition of a substance, the amount of protons and neutrons that a substance has that determines. Avogadro’s constant ensures that the atomic weight on a given element as it ‘cancels out’ the atomic mass unit, amu. Where = number of mole (amount), = number of particles = Avogadro’s constant.
Calculating the number of atoms in molecules
Take the empirical formula of a compound, then take the components of the substance, for take and then multiply them by to get the amount of grams in one mole of the substance.
4.2 Calculating of mass and number of mole
The relative mass, of an element is defined as the weighted mean of the masses of the naturally occurring isotopes, on the scale where carbon-12 is 12 units exactly. Just as the relative atomic mass refers to atoms, relative molecular mass, refers to the molecules. To determine this, take the sum of the relative atomic masses present in the molecule. This is also used for other non-molecule substances as relative formula mass.
Molar mass
The molar mass of a substance, is equal to the relative atomic (or molecular or formula) mass but in terms of .
Calculations involving masses and the mole
Using the molar mass, we can determine the amount (in mols) of a particular substance in a given mass. (Note: when referring to ‘amount’ in chemistry, very often it is in mols)
Where = number of moles (), = mass (), = molar mass ()
Finding the mass of a few atoms or molecules
A technique used in chemistry, unit analysis, is the process of taking several quantities and determining what the output would be by using variables. So formulas can be created to suit the needs of individual questions.
For instance, the two formulas seen so far, and can be used in tandem to create a formula that finds the mass of a few atoms or molecules.
4.3 Empirical and molecular formulas
An application of the mole is determining the formula of newly synthesized compounds. This process involves percentage composition by mass. To determine a compound’s percentage composition by mass, divide the relative atomic mass by the relative molecular mass of the whole compound and express the result as a percentage. Alternatively, if no formula is given, divide the mass of each element by the mass of the whole sample and express the result as a percentage. For example:
The empirical formula is the mole ratio of elements in the compound, it provides only the ratio but doesn’t indicate the actual amount of atoms present in a molecule. The molecular formula of a compound is the actual number of atoms of different elements bonded in a molecule. This is an important distinction as some molecules have the same empirical formula but differing molecular formulas. For instance is the empirical formula for methane, and ethane, .
**The empirical formula of a compound can be determined when given mass proportion using unit analysis.
**The molecular formula of a compound can be determined when given the empirical formula and the molar mass (or relative molar mass), using unit analysis.
4.4 Chemical equations
In chemical reactions, all atoms within a reaction are conserved. For instance in the following reactions, the atoms are only being rearranged neither destroyed nor created. (Anything to the left of the arrow is the reactant, anything to the right is the product.)
The amount of a specific atom on the left must equal the amount of that specific atom on the right. If this is true for all atoms, the chemical equation is balanced, if not it is unbalanced. In order to balance an equation, coefficients must be added so mass is conserved.
Adding symbols to show the physical states of the reactants and products is the final step in writing an equation. They are all written in subscript. (g) represents gas, (l) represents liquid/molten, (s) represents solid, and (aq) represents aqueous (dissolved in a solvent).
The process of balancing equations is known as stoichiometry.
4.5 Mass relationships in chemical reactions
By using both stoichiometry and unit analysis, the mass of any reactant or can be found if the balanced equation and any of the moles, masses etc. of the reactants or products are known. This can be done as when the ratio between all the reactants and products are known, they act as a ‘multiplier’. This process can be represented mathematically as follows:
If the mass of is given:
If the moles of is given :
** = the molar ratio and is the ratio of that can be determined by using the balanced equation. The reciprocal of this can be taken if the units demand it. This method calculated the theoretical yield of the reaction.
Problems involving limiting or excess reactants
When it is explicitly stated that a reagent/reactant is in excess, it is implied that the other reactant(s) are limited. When this is the case, the limiting reagent determines the amount of product. This is akin to wanting to make dough; having only one cup of flour and an unlimited amount of water allows for only one cup of flour’s worth of dough to be made.
Calculating percentage yield
4.6 Factors affecting amounts of gases
Pressure
As gas particles collide with each their container they exert a force on the walls. The force per unit area on the wall is the pressure exerted by the gas.
The SI unit of pressure, is a Pascal. Where , coming from the formula . The IUPAC used to use one atmosphere as a standard, . They now use the bar where .
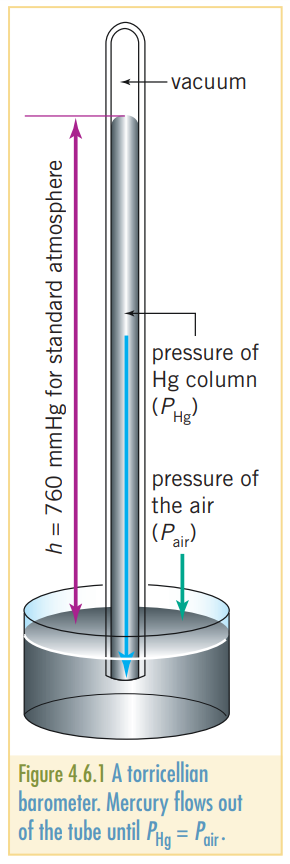
Pressure can be measured using Torricelli’s barometer. By using the height of a column of mercury supported by a gas as a measure of its pressure. The unit, mmHg is names a torr. The atmospheric pressure due to the particles in the atmosphere has a mean value at sea level of 760mmHg. Mathematically, . An alternative to are hectopascals, which are equivalent to .
The amount of pressure on the base of the barometer results in mercury traveling up the barometer. Once at equilibrium, The pressure of the mercury in the column, will be equal to the pressure that the air exerts on the base, . Thus .
Temperature
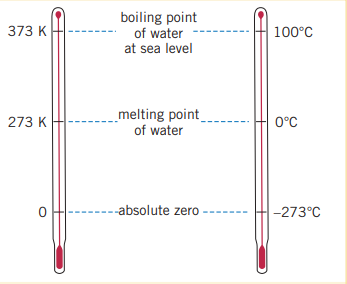
The unit, Celsius is defined by the temperature at which water freezes/ice melts, and the temperature at which it boils/condenses, . An alternative scale, Kelvin, shifts the Celsius scale such that absolute zero, (a temperature at which all non-super fluids freeze) is at 0 instead of . The melting point of water in kelvin is and the boiling point at sea level is .
To convert from Kelvin to degrees Celsius: To convert from degrees Celsius to Kelvin:
Volume
The volume occupied by a gas can be measured by taking the width, length and depth of a container (rectangular prism) and taking the product of all 3.
4.7 Gaseous volume relationships in chemical reactions
In an ideal gas, the gas particles are completely independent of each other, real gases behave less ideally when the pressure of the gas is high. Thus real gases show some deviation from Boyle’s law at pressures greater than 0.
Boyle’s law
The pressure exerted by a given mass of a gas at a constant temperature is inversely proportional to the volume occupied by the gas. Where is the pressure of the gas, is the volume that the gas is occupying and is a constant.
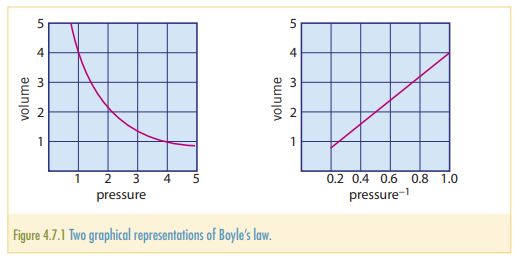
Another application of Boyle’s law is in the following equation: Where is the initial pressure, is the initial pressure , is the final volume, and is the final volume (This applies provided that the amount of gas and temperature stay constant.)
Boyle’s law is consistent with kinetic theory. For instance, if the volume of the container were to be increased, the particles would have to travel further between collisions with the walls. The overall effect of this, would reduce the pressure exerted on the walls of the container. This applies to an ideal gas.
Charles’ law
At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature. *Kelvin is often used as kelvin is never negative and volumes cannot be negative. Where is the volume of the gas, and is the temperature of the gas.
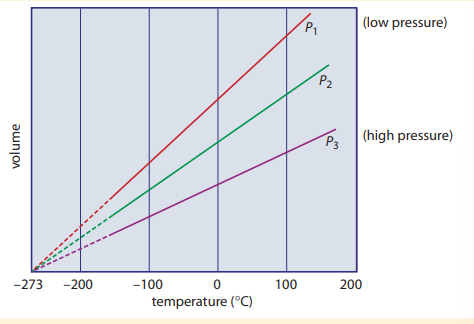
Another application of Charles’ law is in the following equation:
Where is the initial volume, is the initial temperature, is the final temperature, and is the final volume. (This is provided that the pressure and amount of gas is constant.)
Charles’ law is consistent with kinetic theory/ For instance, if the temperature of a gas is increased, the kinetic energy of the particles increases. The particles, moving faster, collides with the container walls more often and with greater force. If the initial pressure is to be maintained, the volume of the container must increase.
Avogadro’s law
Avogadro’s law states that the equal volumes of gases at the same temperature contain equal numbers of particles. This can be represented mathematically as:
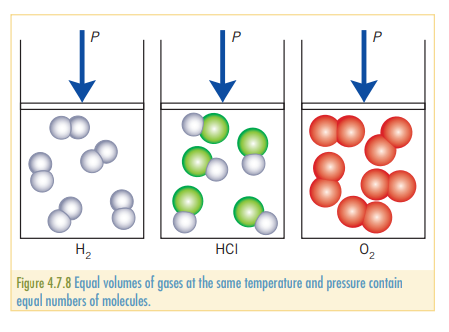
Avogadro’s law can be used to calculate volumes of gases that are made during a reaction. As the volumes of gases under fixed conditions ( and ) are proportional to the number of mole, a molar ratio from an equation can be used to find the ratio of volumes of gases
If the volume of any of the reactants or products are given, and the balanced equation can be found, the volumes of each can be determined via unit analysis. (If there is no volume given, the percentage composition can be found.)
Ideal gases
The ideal gas equation defines the behavior of an ideal gas. Most gases approach this behavior at low pressures. Therefore this equation can be used to determine only one quantity. Another application of the ideal gas law is determining the state of the gas before and after a reaction/change. Where and are the initial and final gas pressures, and are the volumes, and are the temperatures and and are the amounts of gas. Frequently, the amount of gas is constant, so if , the equation above becomes.
Ideal gas equation:
Boyle’s law: at constant and . Charles’ law: at constant and . Avogadro’s law: at constant and \begin{gather} \therefore V \alpha \frac{nT} P \newline V = \frac {nRT} P \newline PV = nRT \end{gather} Where is a constant. This equation is known as the ideal gas equation, or the general gas n equation. Where is the universal gas constant and depends on the units chosen for and (recall that temperature must be in Kelvin.) The most commonly used units are and . Given these units, has a value of . When using the ideal equation, ensure that the units are correct before using the value given.
Molar volume and standard conditions
The molar volume of a gas is the volume occupied by of gas at at . Using the ideal gas equation, this comes out as:
\begin{gather} V = \frac {nRT} P=\frac{1.00mol \times 8.314 \frac {J}{Kmol}\times 273K}{101.3\times 10^3Pa}=0.0224\frac{J}{Pa} \newline =22.4\times 10^{-2}\frac{\frac{kgm^2}{s^2}}{\frac{kg}{ms^2}}=22.4\times 10^{-2}m^3=22.4dm^3 \end{gather}
is the molar volume of a gas under the specified conditions, .
4.8 Solutions
A solvent is the ‘bulk’ substance - for which a solute (the dissolved substance) is dissolved in. When water is the solvent the solution is said to be aqueous. . When mixing salt in water, the solvent is water, the solute is the salt and the solution is the ‘salty water.’ Water is an extremely effective solvent at dissolving polar compounds due to its high polarity. It can be determined qualitatively if a solvent is dissolved in a solution by checking if the solution’s color and opacity are the same as the solvent (before turning into a solution). The concentration of a solution is a quantitative expression of how much solute is dissolved in the solution. Concentration can be expressed in , when concentration is very low, it can be expressed as parts per million, or parts per billion . ** A note that .
Calculating concentration of a solution
The concentration of a solution can be calculated by dividing the amount of solute per unit volume. . Where = the concentration of solution (or other unit), = the number of mole of solution (or other unit) and = volume (or other unit.)
Unit analysis for to of water
Calculating concentrations of components of solutions
In a solution of hydrochloric acid, would have 1 mole of molecules pre of water. As iionizesfully in water, there would the be of ions and of ions. In a solution of ammonium sulfate, ions exist twice as much as and ions.
Dilution of solutions
Dilution is the act of lowering the concentration of a solute in a solvent by adding more of the same solvent. The concentration before and after a dilution can be determined as follows:
. Where is the initial concentration, is the initial volume, is the final concentation and is the final volume.
**If the concentration and volume are give, then the amount of mols can be determined. Then using a balanced equation any other reactants or products can be determined.