2024-07-1617:36 Status:IBnotes Tags: Oil and gas industry
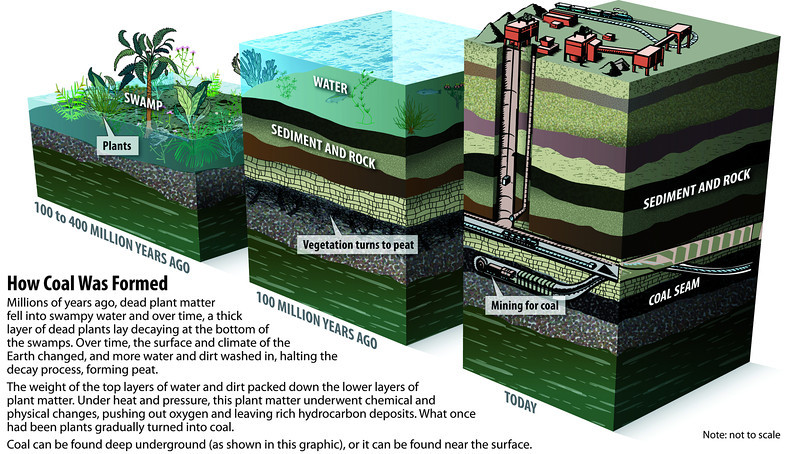
Fossil fuels were formed from the reduction of biological compounds. The partial decomposition of plant and animal matter in hypoxic environments resulted in the production of reservoirs of bitumen, natural gas, and coal seams. Coal is the most plentiful source of solid carbon energy known as anthracite, which began formation in the carboniferous period 286-360 million years ago. The formation of coal and other fossil fuels occurs over millions of years, pressure, and heat.
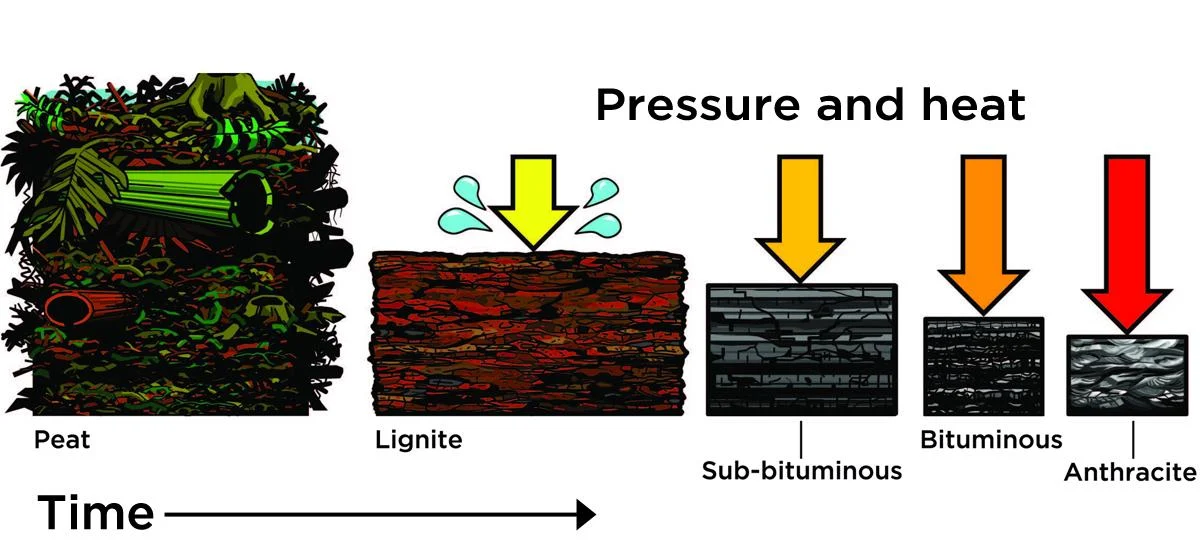
Formation of Coal
Peat can be largely described as buried vegetation in anaerobic conditions = partially decomposed matter. Lignite is the compressed peat under layers of sediment. This compressed peat forms a layer of lignite. The Sub-bituminous coal is further compressed lignite, which forms sub-bituminous and bituminous coal. Anthracite forms after even more pressure and time is put on the (sub-)bituminous coal.
Coal Liquification
Coal liquification uses solid coal, and hydrogen gas, to produce liquid hydrocarbons: Supplies of methane can be increased by cracking or by coal lignification (gasification). Crushed coal is mixed with superheated steam and a mixture of carbon monoxide and hydrogen: Synthesis gas is further processed to make methane by mixing it with and a catalyst: Synthetic natural gas can also be produced by heating crushed coal and steam with catalyst.
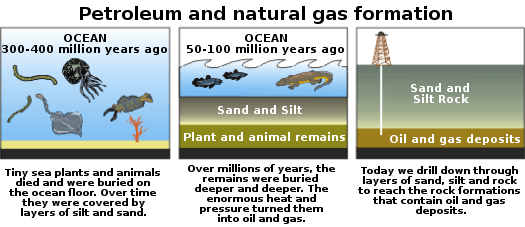
Bitumen (Crude Oil)
Bitumen refers to the unprocessed oil found in reservoirs and is in lesser supply relative to coal, and we use it as a chemical feedstock in addition to energy. Renewable sources are required as eventually reservoirs will deplete. It is easier to produce than, coal as it is often in liquid form, however still needing refinement and distillation for commercial sale.
Refinement of Crude Oil
Because there are many “fractions” of carbon present in crude oil, it needs to be separated from the thick, tar like black fluid it is found naturally as. Crude oil is a mixture of hydrocarbons at different lengths. Because they vary in carbon and hydrogen count (chain lengths), they have differing boiling and melting points. Generally, the longer the chain, the higher the intermolecular force, thus allowing for the longer chains of carbon to remain as shorter chains are evaporated. When gaseous crude oil is pumped into a fractional distillation tube, the cooling gradient forces the hydrocarbons to condense at differing points. This separates crude oil into various kinds of hydrocarbons.
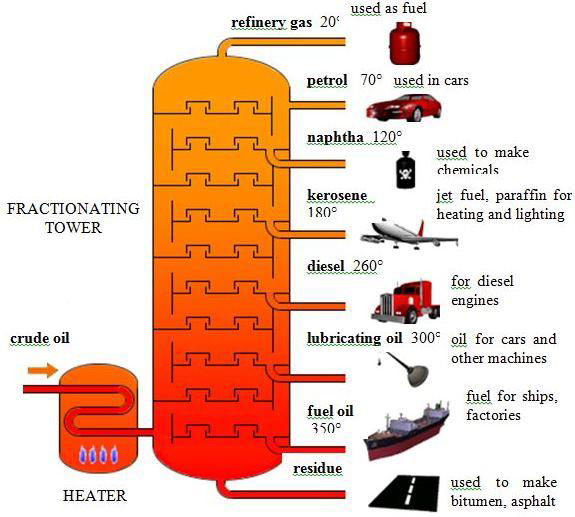
Once fraction of economic benefit are produced, they must be distilled for distribution. Fractional distillation is the process that occurs in refineries to separate important compounds from one another based on their boiling points. The mixture is exposed to high temperatures to vaporize the substances and at regular intervals up the tower, distillation columns at the temperature of the condensation point of the substance lead to storage tanks. The smaller the molecule, the higher the boiling point/condensation point, so the higher up the tower they go before condensation.
Vacuum distillation is used to avoid the high temperatures of fractional distillation (which in turn prevents unwanted thermal cracking of the mixture).
Methane is the cleanest burning of all fossil fuels (as it has the lowest amount of carbon/hydrocarbon molecule, carbon being what turns into carbon dioxide and carbon monoxide). At room temperature, methane is gaseous, allowing for easy transport via pipelines. This requires much capital investment to create, so several places only have propane or even oil as their main means of cooking and heating fuel.
Cracking
Smaller hydrocarbons are easier to ignite and therefore more effective as a fuel than longer chains of hydrocarbons. To make these longer chains into more useful fuels, longer chains are cracked into an alkane and alkene molecule. Although alkanes are better fuels than alkenes, alkenes are still useful in the manufacturing of plastic. For example: (Dodecane to octane and butene), however this is random, and can be broken down into two or more smaller hydrocarbons with the same cumulative carbon and hydrogen atoms as the original chain. There are two methods of doing this:
- Thermal cracking = High temperature and high pressure cracking () breaks the bonds of atoms, this is less popular than catalytic cracking.
- Catalytic cracking = Low temperature, low pressure, with a zeolite catalyst cracking (), producing 5-10 length hydrocarbons (often used for petrol). They rearrange carbocations in order to get specific branches of alkane products at different temperatures.
Efficiency of Fuels
Branched burn more evenly in an engine, and are therefore of higher quality or octane rating. 2,2,4-trimethylpentane (isooctane) is the 100% standard by which all other fuels are measured. High octane number molecules are more compressed, so more power per piston stroke. Heptane is considered to have an octane rating of 0 (maximal knocking). Cyclic structures generally have a higher octane rating than their linear counterparts. Alkenes, have higher octane ratings than the isomeric cycloalkane. Benzene structures also have a high octane rating.
Additional Processes
Catalytic reforming is used to make specific branched alkanes. Alkylation (Addition of an alkane to an alkene) to create a branched chain.
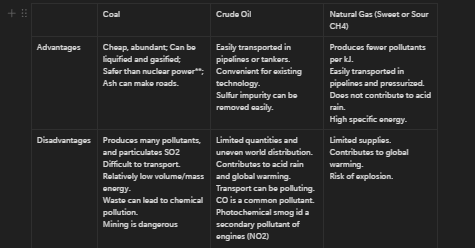
Advantages/Disadvantages
GHGs (Greenhouse Gases)
The media holds great power over populations. Most people do not have a scientific background or the time to adequately research articles posted by media sources. If it looks legitimate, and the presenter appears to be credible, people will believe it. This is a particularly egregious issue in regards to GHGs.
It is widely agreed upon that the rate of GHG production is increasing due to our energy use and lifestyle choices. The temperature of the Earth is maintained by a steady balance between the energy received from the sun and the energy leaving the Earth and going back into space. Incoming radiation is visible and UV wavelengths (short) passes right through our atmosphere. The Earth reflects longer wavelengths, IR which is absorbed by GHGs and causes the bonds to stretch and reverse dipole (like IR spec tests) in the atmosphere. This stretching increases the kinetic energy and is radiated into the atmosphere, space and back to Earth, causing global warming. The term GHG is due to the trapping effect of heating and transmitting UV.
Not all gases absorb IR energy the same. Symmetrical, nonpolar molecules e.g. do not shift diploes and are thus not considered GHGs.
GHF (Greenhouse Factor)
Greenhouse factor, GHF compares the ability of a gas to absorb IR compared to . For instance 10 molecules of water have the same absorption as 1 molecule of carbon dioxide, thus water has a GHF of 0.1; methane is equivalent to 30 molecules of carbon dioxide, thus having a GHF of 30; has a GHF of 160, however, and are not abundant in the atmosphere.
may increase by 100% in 100 years, and the average temperature on Earth may rise in 50 years. This will change crop yields (likely to decrease), change biodistribution (desertification), increase sea levels (which actually offset come of the other effects of a high carbon dioxide concentration such as ocean acidification, however, this causes erratic weather patterns, and likely displacement of people in coastal regions).
Oceanic pH
The ocean’s pH has decreased from 8.2 to 8.1 (as a result of increased GHGs in the atmosphere) which seems small, but keeping in mind that pH is logarithmic, this represents a 26% increase in . The ocean has a heterogeneous equilibrium between and the oceanic pH:
\begin{gather} CO_{2(g)} \rightleftharpoons CO_{2(aq)} \ \ \ \ \ \ \ \ \ \ K_{sp}\uparrow as\ T\uparrow \\ CO_{(aq)} +H_2O_{(l)} \rightleftharpoons H_2CO_{3(aq)} \\H_2CO_{3(aq)} \rightleftharpoons H_3O_{(aq)}^+ + HCO_{3(aq)}^- \\HCO_{3(aq)}\rightleftharpoons H_3O_{(aq)} + CO_{3(aq)} ^{2-} \end{gather}
The oceans also have the benefit of biological populations that can maximize the effect of GHGs. Algae us actually one of the worlds largest carbon sinks as they consume carbon dioxide during photosynthesis. Corals and shellfish incorporate carbonate into their solid shells as calcium carbonate.
Global Dimming
Global dimming is a condition of particles in the air (visual pollutants, and volcanic ash) that scatter light, so less reaches the earth. Incomplete combustion is a large source of particles (soot) common in coal oil and wood burning. Most can be “scrubbed” from chimneys by using binding chemicals. Particles are a nucleating agent for rain drop formation (cloud seeding causes rain/hail to form away from cities to avoid insurance claims).