2024-07-1113:45 Status:IBnotes Tags: Atomic physics American Development of the Atomic Bomb
Recall that all objects radiate EMR. The amount and wavelength depends on the temperature of the body. This reaction of the sun releases all wavelengths (white light). But the sun has a lower temperature “atmosphere: with gases that absorb energy producing absorption lines. These dark bands can be used to identify the elements present. E.g. .
Absorption Lines
Absorption lines correspond to energy absorbed in exciting electrons from lower energy levels to higher energy levels. The blank lines show an ascribed by a substance. As an excited electron from hydrogen falls to its ground state, it emits light. Both emission spectra are perceived from the sun’s corona which supports the fission reaction: . This kind of fusion is different to the one found for the melting of ice for example.
Nuclear Reactions
Nuclear reactions are those in which the nucleons of an atom are changed, so that the chemical types are different in a balance reaction equation: Where in , there are 143 . This is because the mass of uranium and the neutron must be the same as the products mass (this also applies to proton and neutron count). This is a natural process as unstable nuclei decay into more stable atoms. Another example is:
To see more on where the energy comes from see Atomic physics
Nuclear Reactors
How we generate nuclear power; https://www.britannica.com/video/214988/Using-nuclear-power-to-generate-electricity-overview-how-nuclear-power-plants-work
Small modular reactors https://www.youtube.com/watch?v=ldEy6UU8o4A Potential of modular reactors https://www.youtube.com/watch?v=60q_l_VPhtU Mass defect video short https://www.youtube.com/watch?v=yTkojROg-t8
Nuclear Fission
One pellet of uranium 235 (7 grams) can generate as much energy as 4 barrels of oil, 480 cubic meters of natural gas, or 800kg of coal. However, the correct isotope of uranium for nuclear reactors is relatively rare, and needs to be refined. Heavy nuclei will break down into smaller nuclei by releasing energy. When a nucleus is bombarded with a neutron, it splits, a number of neutrons and energy will be released. The neutrons released can then trigger fission of nearby atoms in a chain reaction (bomb).
The products are often radioactive isotopes that can be repurposed in other industries. If neutrons are removed faster than they are produced, the chain reaction can be prevented. Nuclear reactors are designed so that only one neutron from each reaction goes on to initiate another reaction, released energy at a controlled rate and avoiding the massive chain reaction. If a reactor misses, then the reaction can proceed unchecked and could cause a meltdown. There have only been 2 meltdowns.
The chance of a neutron triggering another fission depends on the number of potential nuclei in its path and the speed of the neutrons. Most neutrons travel too fast, so the reactor uses a moderator to slow down the neutrons for absorption.
Control rods (graphite or cadmium) absorb neutrons, preventing the chain reaction, and are added or removed from the reactor to control chain reaction.
Sourcing U-235
Critical mass is the minimum mass of fissionable material needed to sustain a chain reaction. Critical mass depends on the shape, purity, density and consumption.
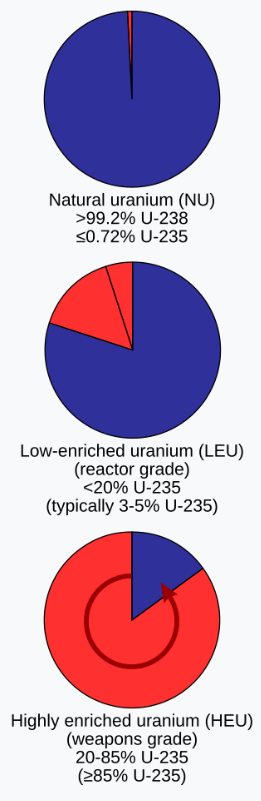
U-238 is not the best fuel as the neutron will most likely be absorbed into U-239. U-235 is the best fissionable fuel, but natural U or has less than 1% U-235 so mist of the fuel must be enriched. There is no easy chemic method for separating the two, but methods exist nonetheless.
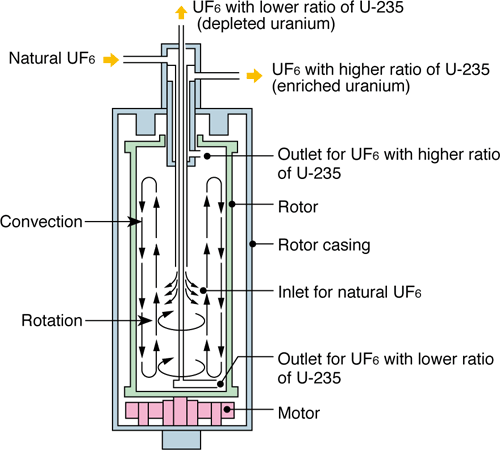
The international atomic energy authority controls fuel enrichment so only few countries can do this. Northern Saskatchewan has several uranium mines, but Canada buys enriched uranium from the US in return for medical grade isotopes produced at Ontario’s Chalk river nuclear facility. Heavier atoms will move more slowly on average than smaller atoms. This principle is used in the gaseous diffusion of , the only volatile ore containing uranium. This method uses a semi-permeable membrane in the following:
is a covalent octahedron (Expanded orbitals) with polar bonds but the molecule is non-polar due to symmetry. This solid sublimes at , so quickly becomes a gas for diffusion. The atoms surrounding the molecule repel other molecules making it easier to sublime. is also a common solid but cannot be used in gaseous diffusion due to ionic bonds, making it a solid with a melting point of , not to mention the boiling point.
Since only a few molecules will separate at any stage, the gas separation processes must be repeated up to 4000 times to get a reasonably pure collection of isotopes.
Graham’s Law
Graham’s law is equated to the kinetic energy of 2 isotopes with slightly different masses. (Where the rate of effusion of a gas is inversely proportional to the root of the molar mass). \begin{gather}E_{kA} = E_{kB} \\ \frac 1 2 m_Av^2 = \frac 1 2 m_Bv^2 \\ \frac {m_A}{m_B} = (\frac {v_B}{v_A})^2 \\ \sqrt {\frac {m_A}{m_B}}=\frac {t_A}{T_B}=\frac {v_B} {v_A} \end{gather}
Centrifuge
One method of isolating U-235 is in the usage of centrifuges. The atomic mass of uranium is 238 so most of the isotopes are heavier than U-235. When the centrifuges are spun, the heavier isotopes, gravitate towards the edge faster than the light isotopes as they are heavier. They then fall towards the bottom of the centrifuge. The light, U-235 stays near the center of the centrifuge, thus isolating the isotope.
Weapons and
Weapons have been designed so that both and , a byproduct of fission reactors can be used. This is one argument people have against nuclear power. They are risky as core meltdowns or terrorist targets. Breeder reactors create from in stepwise negative beta decay. Fast breeder reactions involve bombardment of with neutrons:
Concerns with breeder reactors: is toxic; can be concentrated from reactor grade to weapons grade easily; Breeder reactors are less efficient than uranium and require liquid metal coolant; Could be more susceptible to accidents (if leaked into water for instance); all nuclear reactions create radioactive waste that must be transported and stored. Uranium is not very rake (unlike originally thought) so energy is wasted.
Nuclear Waste
Radioactive isotopes are long lived, meaning they retain their radioactivity for a long time before decaying. Storage of radioactive waste is unlikely to be effective, so scientists are trying to find uses. Every radioactive decay involves the emission of ionizing radiation (alpha radiation, beta radiation, gamma radiation). This removes electrons from matter including biological important molecules like DNA. The ability to ionize water is the threshold that marks the difference between ionizing and nonionizing. Water makes up 70-90% of all living tissue, where the ionization energy of water is . Radiation forms , an extremely reactive free radical, that is a very strong oxidizing agent and will destroy biologically active molecules by moving electrons or hydrogen atoms. These can then produce other free radicals in a chain reaction that will damage DNA, oxidized fatty acids, and amino acids.
High vs. Low Level Waste
Low level wastes have low levels of activity, and short half lives. This come from hospital waste, fuel containers. These require little storage in a cool ed(usually by water) environment.
High level waste have a high activity and long half life. These come from nuclear industry and military. These require remote controlled machinery for safe handling and transport to storage facilities like deep unused mines, and impervious granite in geologically stable areas. U-238 has a half life of years, which means that only half of the U-238 that existed when the Earth formed ahs since decayed away. Some isotopes like have a half-life of a fraction of a second for reference.
*IB only asks for the half lives of 1st order reactions. Each reduction in [RXN] by half will take the same amount of time.
Half-life
Recall from kinetics that half life = time for half of the mass of an isotope to decay; the number of atoms of the isotope to fall to half their original number; or the activity of an isotope to fall to half. Radioactive decay is a first order process, meaning that where is now the decay constant, . So the following formulas take the form:
So when half the original amount of a material exists, half life is independant of the starting concentration. *Do not forget that lambda is negative and k is positive; whether a lot or a little of some material is lost, half-life remains constant.
Energy Storage
Fossil fuels are convenient in that they are a source of potential energy stored in chemical bonds that can be burned in any conditions at any time. Renewable energy sources like solar, wind, wave etc. are only produced in favorable conditions like daytime, optimal winds, consistent tides etc. This means that the energy generated during the day must be stored for later use.
Opponents to renewables often cite the expense and pollution of batteries which have always been the common storage solution of off grid solar applications.
There is another chemical potential energy storage option that is beginning to be well publicized, hydrogen production and storage. Hydrogen is a readily available gas, easily stored and safely maintained, yet opponents will all cite the Hindenburg disaster as a cautionary tale against hydrogen fuel.
Hydrogen is now rated on a color scale based on the source of production. Green hydrogen is all renewably produced, but may also have some questionable materials in the fuel cells and electrolyzers required. Blue (sometimes called gray) hydrogen is produced from another fossil fuel source like methane. Supporters of this gray hydrogen point to their evidence of their commitment to the climate, however there is a superior way to invest in hydrogen production.
Notably Elon Musk was against hydrogen fuel storage as its use in vehicles would directly compete with his Tesla vehicle sales. He claimed that the generation of hydrogen was inefficient but he was basing that on the car producing it own hydrogen. Most on the fly hydrogen relies on the reaction of an alkali earth metal or alkali metal to start it but an alternator in the motor can also drive hydrogen production fueled electric grid as that would support his EV company.
https://www.youtube.com/watch?v=AGTjKJHu99c Hydrogen Hype Dwplanet
https://www.youtube.com/watch?v=j2Qpv1qz-2s Swedish hydrogen house schematics are good
Pressurized water:
In a PWR the primary coolant (superheated water) is pumped under high pressure to the reactor core, then the heated water transfers thermal energy to a steam generator. In contrast to a boiling water reactor, pressure in the primary coolant loop prevents the water from boiling withing the reactor.
The other common US power reactor type is the boiling water reactor (BWR). As the name implies, the inner loop contains both liquid water and steam, which drives the turbines. Since this inner water will contain some radioactive material, this design can cause contamination of the turbine blades.
Fuel enrichment:
A sample of Uranium typically contains 99.2% Uranium-238 and 0.72% Uranium-235. Enriched Uranium contains at least 2-5% Uranium-235, although higher percentages are possible. Nuclear weapons require a sample that contains 99% Uranium-235.
Uranium is enriched through the formation of hexafluoride followed by the separation of Uranium-238 and Uranium-235 in a gas centrifuge. In more detailed terms, a type of Uranium gas is put into a centrifuge — a series of cylinders with a rotor inside of them.
The mixture of isotopes is spun around at high rates (up to 70,000 rev/min). The heavier Uranium-238 requires a larger centripetal force to stay in the circular path and thus moves to the outside of the centrifuge, while the Uranium-235 requires a smaller force and collects together in the center. The Uranium-238 (now called “depleted Uranium”) can then be scraped off the edges while the remaining Uranium is spun a few more times. This process is repeated until the Uranium has the desired percentage of 3% Uranium-235.
The critical mass is the minimum amount of Uranium needed to sustain a controlled reaction. As the surface area to volume ratio of an object increases, the higher the probability of a neutron colliding with it, thus a greater percentage will cause fission. Uranium that contains 20% Uranium-235 has a critical mass of 400 kg.