2024-07-1617:23 Status:IBnotes
Overview
Kinetics is the study of reaction rates or changes in the concentration of reactant and products as a function of time.
Mechanism
A mechanism is defined as a set of stepwise changes that occur as reactants undergo their conversion to products. This is primarily done by communicating the transfer of (valance, lone pair, and & bonds) electrons.
Factors Affecting Reaction Rate
Under a given set of conditions, each reaction has its own characteristic rate determined by the chemical nature of its reactants. E.g.
= Very fast = Very slow
KMT (Kinetic Molecular Theory)
All materials are made up of particles, that are in constant motion. A reaction may occur when reactant properties collide with each other (=Collision theory): Reactions may occur when particles collide but the vast majority of collisions so not result in a reaction. Instead, the following conditions must be met:
- Reactant particles must physically collide.
- Reactant particles must have sufficient energy.
- Reactant particles must collide in the correct orientation.
5 Factors to Increase the Rate of Reaction
- The nature of the reactants Some materials react faster than others.
- Temperature Colliding particles must have a certain amount of kinetic energy in order to react, thus increasing the of the particles. This entails more particles with more energy to overcome this “activation energy”
- Concentration (pressure) The more particles present, the more collisions that will occur.
- Physical state (SA, Agitation/stirring) A faster reaction occurs when there is more surface area available. Thus when more finely divided the faster the particles react.
- Catalyst (Enzymes) The catalyst is a material that increases the rate of reaction because it is able to correctly orientate the molecule.
Reaction Rate
The reaction rate is the change in concentration for a reactant or product over a period of time.
Where rate has the units of .
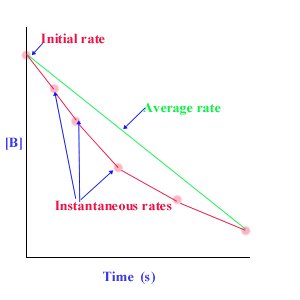
Plotting/measuring rate
- The initial rate is determined by taking the tangent’s slope at time = 0.
- The instantaneous rate = draw a tangent at the specific time and take the slope.
- The average rate = connect two endpoints and take the slope.
The general relationship for reaction progression is:
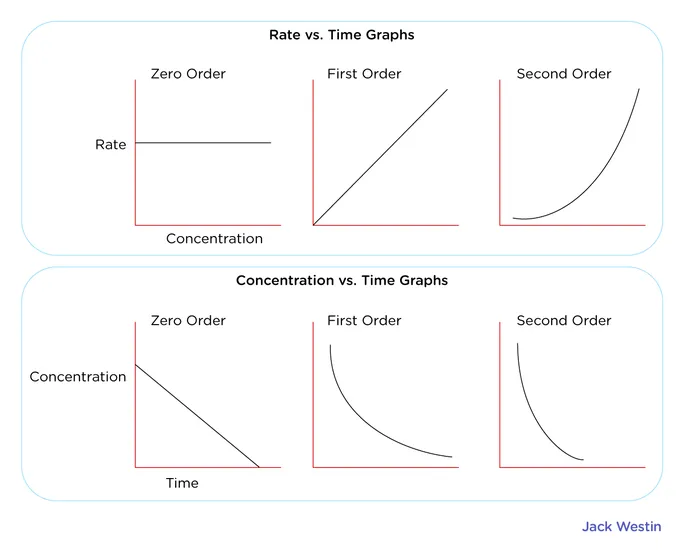
- For a non-zero reaction, the rate = or rate = .
- Thus the rate constant and concentration of the reactant(s) decrease in a linear fashion.
Graphical Relationship
For a zero-order reaction, the rate is expressed as rate = or rate = . This relationship is constant, and the concentration of the reactant(s) decrease in a linear fashion.
For a first order reaction, rate = or rate = . For a second order reaction, rate = .
Half-life for first order reactions
The reaction of half-life is the time required for the reactant concentration to reach half of its original value. Half life is a characteristic of a reaction at given temperatures.
- Radioactive decay - follows first order process.
- Iodine-131, has a half-life of 8 days.
Rate Law
The rate law is an expression that incorporates the concentration of reactant, [reactant], or concentration of product, [product] with temperature. The rate law is determined empirically. For our studies we will only consider the reactants. Where is the reaction constant (can be negative), and are the reaction orders.
is a specific value for each reaction. It does not change based on the concentration of the reactants, products nor with time. Instead it is dependent on only temperature and catalysts present. The units of change depending on the reaction order ( exists to also cancel out units).
The rate of reaction is a function of the number of the number of collisions and the effectiveness of each collisions:
Activation Energy and Maxwell-Boltzmann Distribution.
Arrhenius suggested that every reaction has an energy threshold that the colliding molecules must have in order to react: The minimum energy requirement is in the activation energy, . Breaking bonds requires energy, and bonds must be broken before they can be rearranged and “start” the reaction.
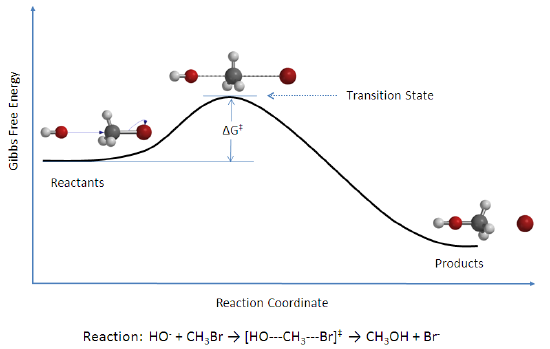 (Image of: activation energy breaking bonds Arrhenius)
(Image of: activation energy breaking bonds Arrhenius)
Particles colliding with less kinetic energy than the will never react because the bonds won’t break. Thus particles with will have their bonds break and this may cause a reaction.
Maxwell-Boltzmann Distribution
A Maxwell Boltzmann distribution is a graph that plots the kinetic energy of gas particles against their numbers. There is a random variation with some particles having low and some having very high . All particles with , may react when they collide (even if the average temperature suggests they should not).
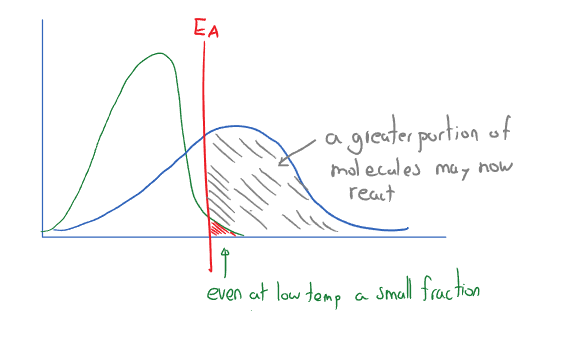

 At a higher temperature, a greater portion of molecules have . Think of a cup and how fast the water evaporates, then how fast water evaporates at .
At a higher temperature, a greater portion of molecules have . Think of a cup and how fast the water evaporates, then how fast water evaporates at .
Activated Complex (transition state)
This model explains what happens when we have enough .
- If temperature increases, the reaction rate increases as there are more collisions have
- At a given temperature, it is possible to compare two reactions. The reaction with the higher , will have fewer effective collisions. a. When an effective collision occurs, the molecules enter the activated complex. i. An activated complex can refer to ( transition state, intermediates, or any of the intermediate (elementary) steps seen in organic mechanisms.).
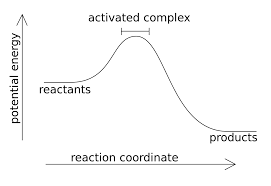
Temperature’s relationship to .
The temperature change effects the rate constant, . This is expressed in the Arrhenius equation:
Where is the rate constant; is the base for natural log; is the universal gas constant, ; is the absolute temperature , is the activation energy , is the collision frequency (the number of particle collisions), is the orientation probability factor (a number between 0 and 1) that relates the probability that two colliding particles will be oriented correctly and will react. * Th closer this value is to 1, the better. Often, and are combined into the factor, . Note: As temperature increases, will become larger (rate increases). This can be manipulated to be: which is a linear equation in the form . * The following formula may also be used to measure a change in rate: .
Measuring and Describing Rate with Theory
Rate of a reaction is a function of the number of collisions and the effectiveness of each collision. The reaction rate can be calculated by:
Collision frequency = Concentration, surface area, temperature. Fraction effective = Nature of reactant, catalyst, temperature. Reaction rate is measured by:
- Time (s)
- Flame test
- pH/pOH
- Boiling point/Melting point
- Conductivity
- Specific heat
- Titration
- Mass
- Volume of gas
- Chromatography RGB, hex codes
- Intensity
- Saturation
- Precipitation
- Smell
- Viscosity
- Radioactivity
- Pressure
- Light
- Magnetic field
- Color spectrometer
Reaction Mechanism
Organic mechanisms and reactions An overall balanced equation does not show as what actually happens during a reaction. Reaction mechanisms are series of steps that occur sequentially that add up to the overall reaction.
Reaction mechanisms (as to occur nearly instantaneously), are theoretical expression and can be tested by hard to truly prove. However, they follow some principles:
- Each step is only the collision of two particles.
- Any products formed and then reacted is called a reaction intermediate.
- Reaction intermediates are unstable (especially compared to reactants and products).
- The intermediates must exist for long enough to go to the next step.
- Each step is referred to as an elementary step (each collision).
- All intermediates must cancel out.
- A reaction involving one particle is unimolecular.
- A reaction involving two particles is bimolecular.
Example 1
Overall reaction: Step 1. Step 2. Step 3.
Example 2
Overall reaction: Step 1. - Unimolecular rate = Step 2. - Bimolecular rate = . The rates can be combined to create the overall rate, however, this does not include the intermediate.
Rate Determining Step (RDS)
Recall that the rate law for a reaction must be determined experimentally. However, for elementary steps, only we can use the coefficients for the reaction order.
.
- Elementary steps…
- Elementary steps need to be physically reasonable: unimolecular, bimolecular.
- The reaction mechanism must match the empirical rate law.
- The rate determining step: the overall reaction can only be as fast as the slowest elementary step. (As the reaction is ‘waiting’ for the slowest elementary step to commence for the rest of the reaction to proceed).
Example 3
Overall reaction: The expected rate law: vs. The empirical rate law: . The reason for this is: Step 1: rate= = RDS. Step 2: rate= Overall reaction: rate is that of the RDS = . When reviewing a proposed mechanism, the overall reaction rate should not include intermediates. The rate law includes the RDS and all of the steps before it.
Rate
Step 1: Substrate → Carbocation (slow), RDS= Step 2: Carbocation + :Nu → Product (fast) Overall rate =
Rate
Substrate + :Nu → Transition RDS = Transition → Product (does not really have a rate)
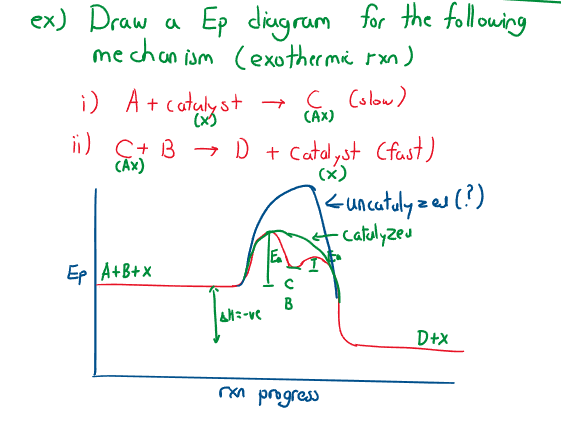
Reaction mechanisms and graphs
For the reaction:
- (slow reaction) RDS
- (fast reaction)
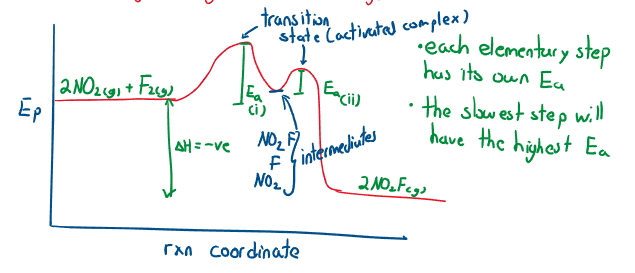 Experimentally, and create one ‘hump’ instead of two distinct ones, only one is seen (the second reaction is reduced to one hump).
Experimentally, and create one ‘hump’ instead of two distinct ones, only one is seen (the second reaction is reduced to one hump).
Catalysts
A catalyst is a substance that increases the rate of reaction, without being consumed. They provide alternate pathways to that of uncatalyzed reactions: this makes the constant increase, but does not increase the yield.
Heterogeneous catalyst
These generally occur as a solid/separate phase as the reaction (e.g. catalytic converter)
Homogeneous catalyst
These generally occur in solution/the same phase as the reaction (e.g. in esterification)